Editor: Nina
Scientists have developed an iron-based nanovehicle that delivers the ferroptosis-inducing molecule Fin56 and responds to both acidic tumor environments and near-infrared (NIR) light. This platform achieves targeted, synergistic therapy against osteosarcoma by combining drug delivery, chemodynamic therapy (CDT), and photothermal therapy (PTT) to amplify ferroptotic cell death, offering a promising strategy for treating drug-resistant bone tumors.
Key Preview
- Research Question: How can an iron-based nanovehicle boost ferroptosis therapy to overcome the limitations of current osteosarcoma treatments?
- Research Design and Strategy: The study employed a novel nanovehicle (FSR-Fin56) that combines chemodynamic therapy (CDT) and photothermal therapy (PTT) to enhance the therapeutic efficacy against osteosarcoma through targeted delivery and localized hyperthermia.
- Method: The researchers synthesized an iron oxide-based nanovehicle modified with RGD peptides to selectively target osteosarcoma cells. The effectiveness of FSR-Fin56 was evaluated through in vitro and in vivo experiments, assessing its ability to induce ferroptosis under hyperthermic conditions.
- Key Results: The FSR-Fin56 nanovehicle demonstrated significant therapeutic efficacy, leading to enhanced cell death in osteosarcoma models through the induction of ferroptosis, while exhibiting excellent biocompatibility.
- Significance of the Research: This study represents a breakthrough in cancer therapy by utilizing a dual-action nanovehicle, addressing the critical challenge of apoptosis resistance in osteosarcoma treatment and offering a promising avenue for future oncological therapies.
Background: Overcoming the Limits of Conventional Osteosarcoma Therapies
Osteosarcoma is the most common primary malignant bone tumor, primarily affecting children and adolescents. Despite improvements in surgery and chemotherapy, the survival rate for patients with recurrent or metastatic osteosarcoma remains low. A key challenge lies in the tumor’s resistance to apoptosis, the typical form of programmed cell death targeted by existing chemotherapeutic agents.
In recent years, ferroptosis has emerged as a promising alternative. It is a distinct form of regulated cell death driven by iron-dependent lipid peroxidation and excessive ROS generation. Unlike apoptosis, ferroptosis disrupts cellular redox homeostasis, providing a mechanism to eliminate tumor cells that resist traditional therapies. However, ferroptosis inducers such as Fin56 face two significant barriers in clinical application: (1) insufficient induction of ferroptosis due to high levels of GSH and GPX4 in tumor cells, and (2) poor tumor selectivity and systemic toxicity of small-molecule drugs.
Therefore, a system that allows targeted, controlled, and amplified delivery of ferroptosis-inducing agents is urgently needed to overcome these challenges and improve treatment outcomes.
Research Content: A Multifunctional Nanovehicle to Induce Ferroptosis
To address the limitations above, the researchers designed a novel nanoplatform named FSR-Fin56. This nanovehicle integrates four functional components: an Fe₃O₄ core for iron supply and photothermal heating, a mesoporous silica shell for drug loading, RGD peptides for active tumor targeting, and Fin56 as the ferroptosis trigger. The vehicle is engineered to release its contents in response to acidic pH and NIR light, both characteristic of the tumor microenvironment.
Design Rationale and Components:
- The Fe₃O₄ nanoparticle core serves as both a Fenton catalyst and a photothermal agent. It can convert endogenous hydrogen peroxide into toxic hydroxyl radicals and generate heat upon NIR irradiation.
- The mesoporous silica shell enables high loading of Fin56 and ensures stability during systemic circulation.
- RGD peptides on the surface target integrin receptors overexpressed in osteosarcoma cells, facilitating active tumor homing.
- NIR-triggered release enables spatiotemporal control over drug delivery and enhances local therapeutic efficacy.
Therapeutic Strategy: Multi-Modal Synergy
This nanoplatform leverages a multi-modal approach to amplify ferroptosis:
1.Controlled Drug Release: The mesoporous silica shell remains stable in neutral environments but rapidly releases Fin56 under acidic conditions. This release is significantly accelerated by NIR-induced heat, ensuring on-demand drug activation.
Figure 1 The release proffles of Fin56 from FSR-Fin56 in PBS at various pH values with NIR irradiation or not.
2.Redox Collapse: The Fe₃O₄ core catalyzes Fenton reactions in the presence of H₂O₂ and acidic pH, leading to substantial ROS accumulation and GSH depletion in tumor cells. These processes compromise cellular antioxidant defenses.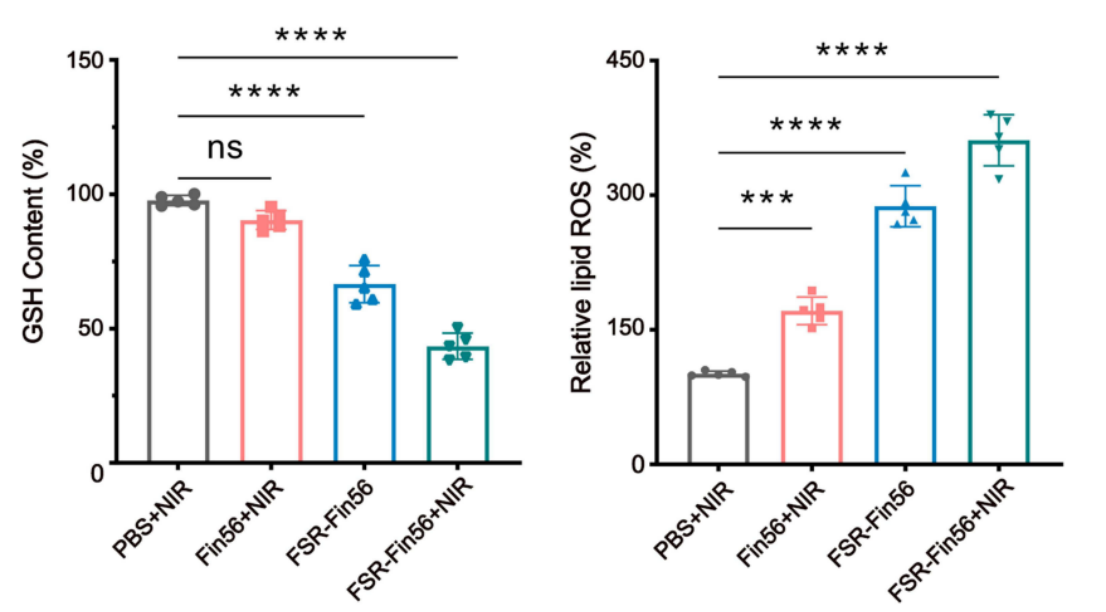
Figure 2. Relative cellular GSH (F) and LPOs (represented by MDA content) (G) levels of MNNG/HOS cells after corresponding treatment (***P<0.001, ****sP
3.GPX4 Inhibition: Fin56 contributes by degrading GPX4, a key enzyme that protects cells from lipid peroxidation. GPX4 loss triggers massive accumulation of lipid ROS, a hallmark of ferroptosis.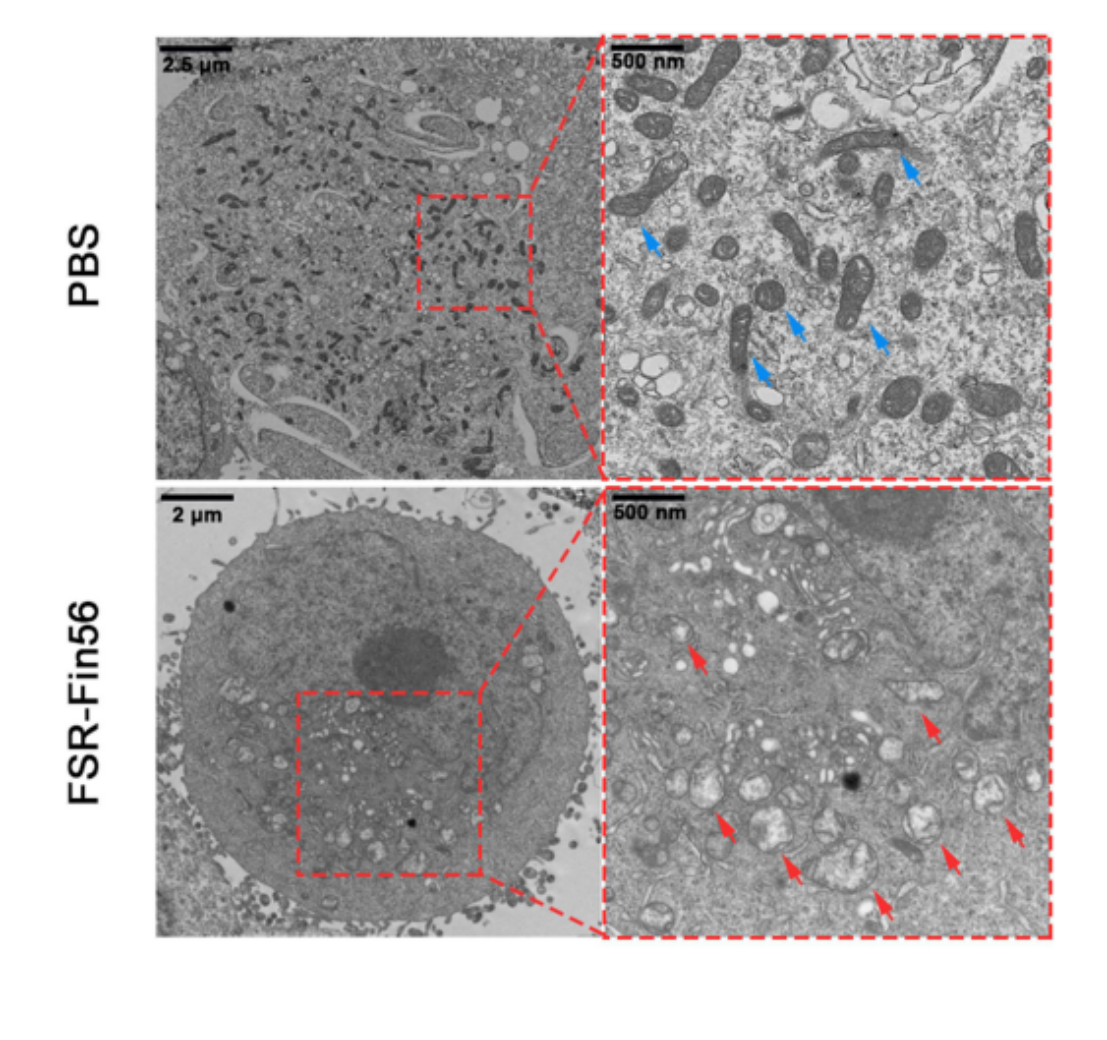
Figure 3. TEM images of MNNG/HOS cells after different treatments. The blue and red arrows indicated relative normal and damaged mitochondria, respectively.
4.Photothermal Enhancement: Under 808 nm NIR irradiation, the Fe₃O₄ nanoparticles produce local heat that enhances Fin56 release and accelerates catalytic efficiency. This not only increases therapeutic potency but also allows external modulation of treatment timing.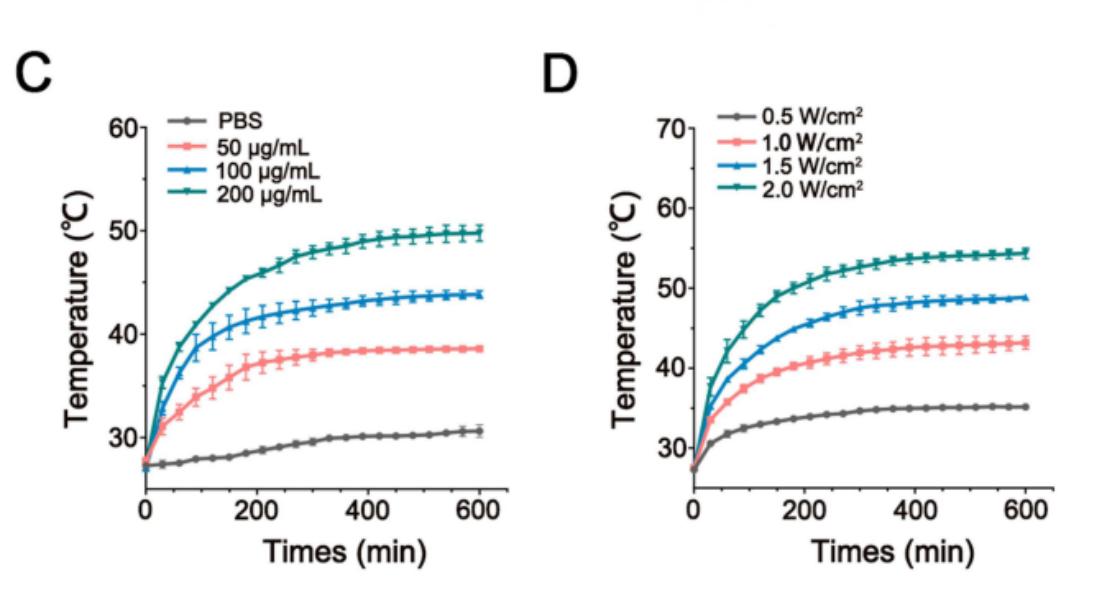
Figure 4. Time-dependent temperature varieties of FSR under 808 nm laser irradiation with different concentrations © or different power densities (D).
Experimental Validation: Proof of Concept
To verify the concept, the authors conducted a series of in vitro and in vivo experiments, focusing on a few key evaluations:
- Drug Release Kinetics: Fin56 release from the nanovehicle was pH- and NIR-dependent. More than 70% of the drug was released within 24 hours under pH 5.0 and NIR irradiation, compared to less than 25% under normal physiological conditions.
- Ferroptosis Indicators: ROS and lipid peroxidation levels were significantly elevated in osteosarcoma cells treated with FSR-Fin56 + NIR, confirming disruption of redox balance.
- Tumor Accumulation and Heating: In vivo imaging showed strong accumulation of ICG-labeled nanovehicles at the tumor site. Upon NIR irradiation, tumor temperatures increased to ~45°C, sufficient to activate ferroptosis pathways.
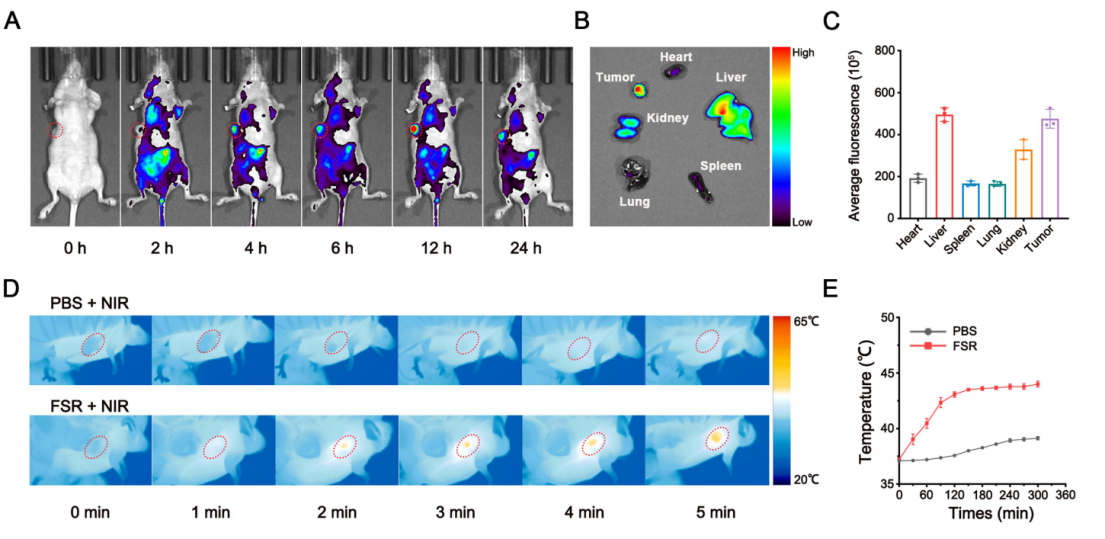
Figure 5. Biodistribution and photothermal effect of FSR NPs in vivo.
- Antitumor Efficacy: In osteosarcoma-bearing mice, the FSR-Fin56 + NIR group exhibited nearly complete tumor growth inhibition. Tumor volumes were significantly reduced compared to other control groups.
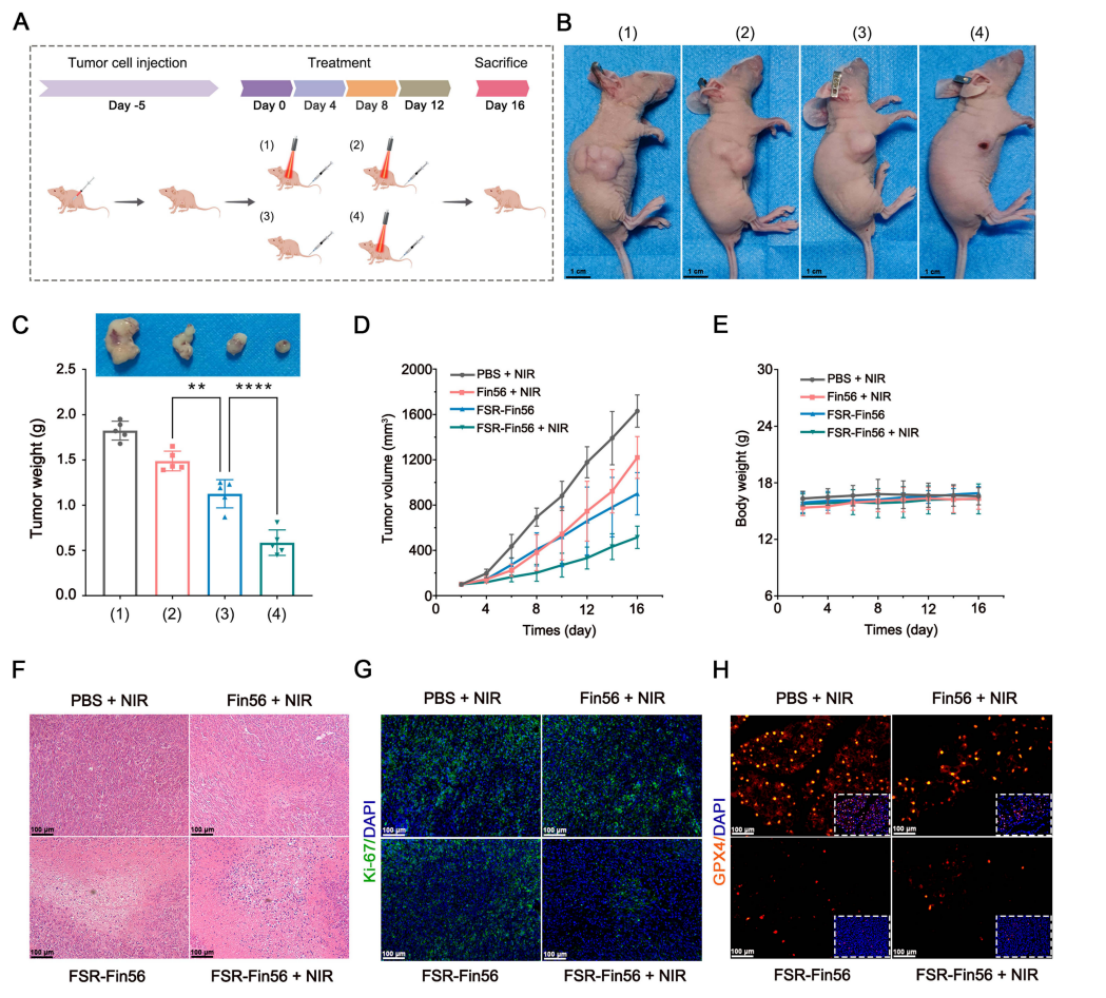
Figure 6. In vivo antitumor effect.
No notable toxicity was observed in major organs, and liver and kidney function remained within safe ranges, supporting the platform’s biosafety.
Conclusion
This study introduces a novel nanoplatform capable of overcoming key limitations in ferroptosis-based cancer therapy. By combining an iron-rich catalytic core, a controlled drug release system, tumor-targeting peptides, and external photothermal activation, the FSR-Fin56 nanovehicle offers a highly selective, potent, and safe approach to treat osteosarcoma.
The results not only validate the efficacy of combining CDT and PTT to amplify ferroptosis but also provide a generalizable strategy for applying ferroptosis therapy to other solid tumors. The use of pH/NIR dual responsiveness, coupled with active targeting and redox pathway collapse, represents a major step forward in smart nanomedicine design.
As ferroptosis gains recognition as a powerful antitumor mechanism, platforms like FSR-Fin56 will likely play a crucial role in the development of next-generation cancer therapeutics.
Reference
Zhang, Yiran, et al. “Iron-Based Nanovehicle Delivering Fin56 for Hyperthermia-Boosted Ferroptosis Therapy Against Osteosarcoma.” International Journal of Nanomedicine, vol. 19, 2024, pp. 91–107. Dove Medical Press, https://doi.org/10.2147/IJN.S441112.
