Editor: Nina
Scientists develop an intranasal vaccine utilizing cationic crosslinked carbon dots-adjuvanted receptor-binding domain (RBD-HR) to induce protective immunity against Omicron variants of SARS-CoV-2.
Key Preview
Research Question: The study investigates whether an intranasal vaccine formulated with cationic crosslinked carbon dots (CCD) can effectively induce protective immunity against Omicron variants of SARS-CoV-2.
Research Design and Strategy: Researchers developed an intranasal vaccine containing CCD and a SARS-CoV-2 antigen known as RBD-HR. They aimed to assess the vaccine’s immunogenicity and its ability to activate both systemic and mucosal immune responses.
Method: The study involved immunizing female BALB/c mice and rabbits with CCD/RBD-HR followed by challenges with the Omicron variant. The researchers measured antibody responses, neutralization capacity, and T-cell activation through various assays.
Key Results: The intranasal CCD/RBD-HR vaccine induced high levels of serum IgG antibodies and robust mucosal IgA responses. Importantly, it provided complete protection against Omicron infection, with almost undetectable viral RNA in immunized mice.
Significance of the Research: This research signifies a potential breakthrough in developing effective mucosal vaccines for COVID-19, particularly in addressing the challenges posed by emerging SARS-CoV-2 variants.
Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has profoundly impacted global health, economies, and societies. Emerging in late 2019, SARS-CoV-2 has undergone several mutations, leading to the rise of variants such as Omicron, which exhibit significant immune escape capabilities. These variants have raised concerns regarding the effectiveness of existing vaccines and therapeutic interventions, necessitating the development of innovative strategies to combat the disease.
Traditional vaccine strategies for respiratory viruses typically involve intramuscular (IM) administration of inactivated or live-attenuated viruses, recombinant proteins, or viral vectors. This method aims to induce systemic immunity by generating neutralizing antibodies and activating T-cell responses. However, while IM vaccines can elicit robust systemic immunity, they often fail to generate sufficient mucosal immunity in the respiratory tract, which is crucial for blocking viral entry and transmission. This limitation is particularly problematic for respiratory viruses, as they primarily infect mucosal surfaces, allowing for continued transmission even among vaccinated individuals. The lack of effective mucosal responses can lead to increased susceptibility to infection and the emergence of new variants, undermining vaccination efforts.
The challenges associated with traditional vaccine delivery strategies highlight the urgent need for innovative approaches that can effectively stimulate both systemic and mucosal immunity. One promising strategy involves the development of mucosal vaccines that can be administered intranasally, targeting the site of viral entry directly. This approach not only aims to enhance local immune responses but also seeks to improve overall vaccine efficacy against circulating variants. The use of novel adjuvants, such as cationic crosslinked carbon dots (CCDs), is instrumental in this innovative delivery strategy. These adjuvants can enhance the immunogenicity of the antigen, promote sustained release, and facilitate antigen uptake by mucosal epithelial cells, ultimately leading to a more effective immune response. By leveraging these advancements in drug delivery technology, researchers aim to address the limitations of current vaccine strategies and provide more robust protection against COVID-19 and its variants.
Research Team and Aim
The research team behind this innovative study is led by Hong Lei, alongside key contributors Aqu Alu, Jingyun Yang, Xi He, and several other collaborators from the State Key Laboratory of Biotherapy and Cancer Center at Sichuan University. The study was conducted between January 2023 and April 2023, culminating in the publication of the paper titled “Cationic crosslinked carbon dots-adjuvanted intranasal vaccine induces protective immunity against Omicron-included SARS-CoV-2 variants” in the esteemed journal Nature Communications.
The primary aim of the research, as articulated by lead researcher Hong Lei, is to develop an effective intranasal vaccine that combines cationic crosslinked carbon dots (CCDs) with the receptor-binding domain (RBD-HR) antigen of SARS-CoV-2. The goal is to assess the vaccine’s ability to induce robust systemic and mucosal immune responses, thereby providing complete protection against the Omicron variant of the virus. This innovative approach seeks to address the limitations of traditional vaccines in eliciting strong mucosal immunity, which is crucial for combating respiratory infections.
Experimental Process
Experiment 1: Preparation of CCD/RBD-HR Nanoparticles
Primary Technique:
The main method employed in this study is the synthesis of cationic crosslinked carbon dots (CCDs) and their subsequent combination with the SARS-CoV-2 receptor-binding domain (RBD-HR) antigen to form nanoparticles for intranasal delivery.
Key Steps:
- Synthesis of CCDs: Cationic carbon dots were synthesized using branched polyethylenimine (PEI 1.8 kDa) as a precursor. PEI was dissolved in dried ethanol, followed by the addition of hydrogen peroxide, and heated in a Teflon autoclave at 185 °C for 5 hours. The resultant product was filtered and vacuum-dried.
- Linker Reaction: The linker compound was synthesized and then reacted with the CCDs via a ring-opening reaction to create crosslinked carbon dots. This step was crucial for enhancing the interaction between CCD and RBD-HR.
- Formation of Nanoparticles: The synthesized CCDs were mixed with the RBD-HR antigen in a mass ratio of 10:1 (CCD to RBD-HR) at room temperature for 30 minutes, allowing for electrostatic interactions to form stable nanoparticles.
Data Collection and Analysis:
Characterization of the nanoparticles was conducted using transmission electron microscopy (TEM) to observe their morphology and size, while zeta potential measurements were taken to assess surface charge. The loading capacity of RBD-HR within CCDs was quantified through a BCA Protein Assay after centrifugation.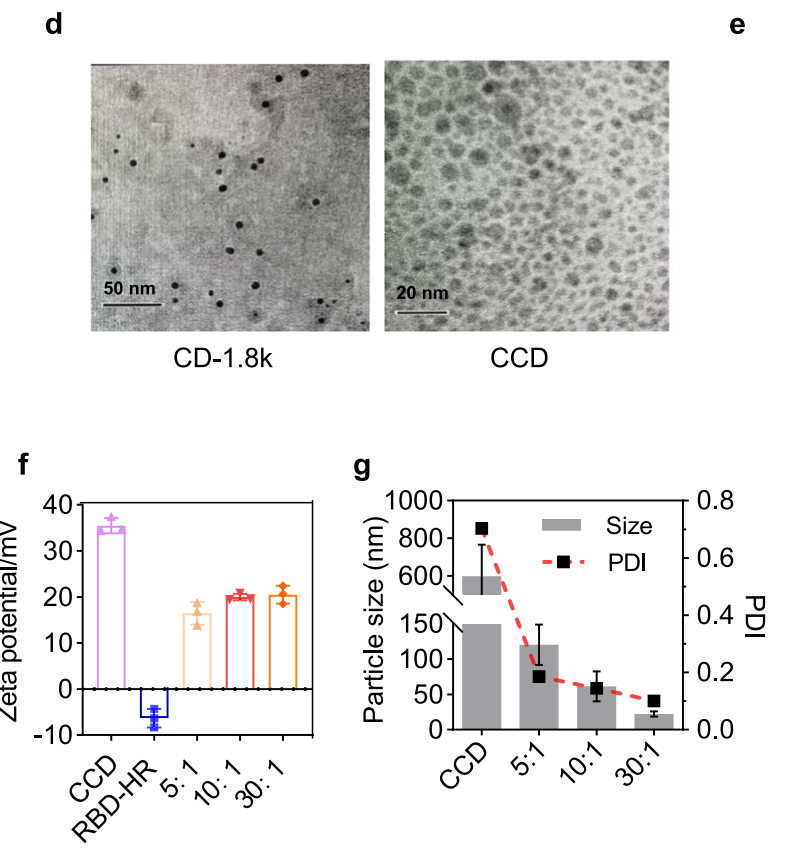
Figure 1. d. The morphology of CD-1.8k (left) and CCD (right) observed by TEM. f, g The zeta potentials (f) and particle sizes (g) of CCD/RBD-HR with different mass ratios.
Result:
The resulting CCD/RBD-HR nanoparticles displayed an average particle size of approximately 60 nm, with a zeta potential of +20 mV, indicating good stability and potential for cellular uptake.
Novel Aspect:
This experiment highlights the innovative use of CCDs as an adjuvant in forming nanoparticles with RBD-HR, which enhances the immunogenicity of the vaccine compared to traditional delivery systems that often lack effective adjuvants. The use of CCDs also improves the stability and bioavailability of the antigen, facilitating mucosal delivery.
Experiment 2: Immunization Protocol and Immune Response Assessment
Primary Technique:
The primary technique for this experiment involved intranasal immunization of female BALB/c mice and subsequent assessment of immune responses through serum and mucosal sample collection.
Key Steps:
- Immunization Schedule: Mice were divided into groups and intranasally immunized with either CCD/RBD-HR nanoparticles, naked RBD-HR, or PBS as control on days 0, 14, and 28.
- Sample Collection: On day 35, blood samples were collected via retro-orbital bleeding, and bronchoalveolar lavage (BAL) fluid was collected for analysis of antibody responses.
Data Collection and Analysis:
Serum antibody levels (IgG and IgA) were quantified using enzyme-linked immunosorbent assay (ELISA). The neutralization capacity of the serum was evaluated against authentic SARS-CoV-2 variants and pseudoviruses using a live virus neutralization assay.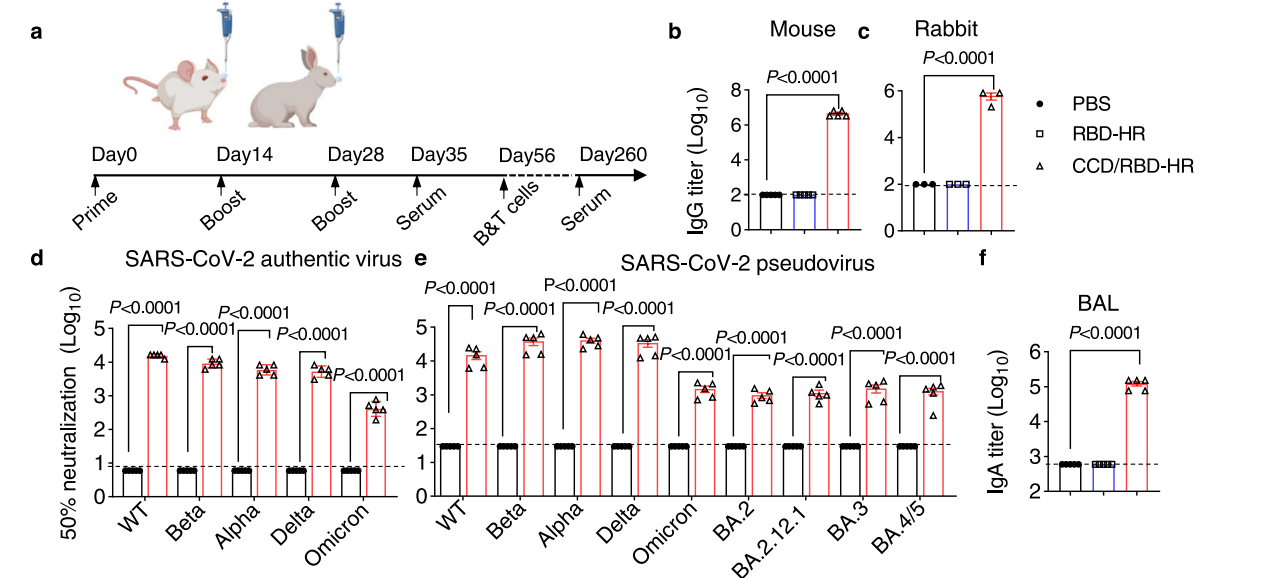
Figure 2. b, c Serum anti-RBD-HR IgG titers in immunized mice (b) and rabbits © on day 35. d, e Neutralizing of authentic viruses (d) and pseudoviruses (e) of SARS-CoV-2 by the immune sera on day 35
Result:
The results indicated that mice immunized with CCD/RBD-HR exhibited significantly higher serum IgG titers and mucosal IgA responses compared to controls. Notably, the immune sera demonstrated broad neutralization ability against various SARS-CoV-2 variants, including Omicron.
Novel Aspect:
This experiment underscores the effectiveness of intranasal administration of CCD/RBD-HR in inducing robust systemic and mucosal immune responses, a significant improvement over conventional intramuscular vaccination strategies that often result in weaker mucosal immunity.
Experiment 3: Viral Challenge and Protection Assessment
Primary Technique:
The primary technique employed in this experiment was a viral challenge study to evaluate the protective efficacy of the CCD/RBD-HR vaccine against SARS-CoV-2 Omicron infection.
Key Steps:
- Viral Challenge: After the completion of the immunization schedule, mice were challenged intranasally with 5 × 10^5 TCID50 of the SARS-CoV-2 Omicron variant.
- Sample Collection Post-Infection: Mice were euthanized 4 days post-infection, and tissue samples (nasal turbinates, trachea, and lungs) were harvested for viral RNA quantification.
Data Collection and Analysis:
Quantitative viral RNA levels were measured using reverse transcription quantitative polymerase chain reaction (RT-qPCR). Histopathological evaluations of lung tissues were performed following standard staining protocols.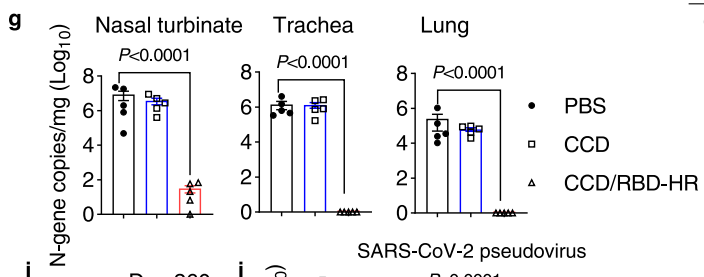
Figure 3. g. Viral RNA levels in the nasal turbinates, trachea, and lungs at 4 dpi.
Result:
Mice immunized with CCD/RBD-HR displayed nearly undetectable levels of viral RNA in the respiratory tract, indicating complete protection against Omicron infection, while control groups showed significant viral loads and inflammatory responses.
Novel Aspect:
The study’s design showcases the novel capability of the CCD/RBD-HR vaccine to confer robust protection against a challenging viral variant, emphasizing its potential as an effective mucosal vaccine platform in comparison to current vaccines that primarily target systemic immunity without strong mucosal efficacy.
Experiment 4: Immune Memory and Longevity Assessment
Primary Technique:
The primary technique for this experiment was the evaluation of immune memory in mice over an extended period following vaccination.
Key Steps:
- Longitudinal Study Design: Mice were immunized as previously described and monitored for immune response longevity, with blood samples collected at 35 days and again at 260 days post-immunization.
- Antibody Measurement: Serum samples were analyzed for RBD-HR-specific IgG levels and neutralization capacity against SARS-CoV-2 variants.
Data Collection and Analysis:
ELISA was employed to quantify IgG titers, and neutralization assays were conducted to evaluate the functional activity of the immune sera.
Result:
The findings indicated that RBD-HR-specific IgG levels remained significantly high at 260 days post-immunization, with preserved neutralization capacity against both ancestral and variant strains of SARS-CoV-2.
Novel Aspect:
This experiment highlights the long-lasting immune response generated by the CCD/RBD-HR vaccine, which is a notable advantage over traditional vaccines that often exhibit waning immunity over time. This aspect of longevity is crucial for developing effective vaccination strategies in response to emerging variants.
Conclusion
The successful development of the intranasal vaccine utilizing cationic crosslinked carbon dots (CCDs) as an innovative drug delivery system represents a significant advancement in the fight against COVID-19, particularly against emerging variants like Omicron. This achievement was realized through a meticulous process that combined the synthesis of CCDs with the receptor-binding domain (RBD-HR) antigen of SARS-CoV-2, resulting in stable nanoparticles capable of eliciting strong immune responses. The formulation not only demonstrated enhanced immunogenicity but also effectively targeted mucosal surfaces to induce both systemic and local immunity.
The highlights of this study include the induction of high levels of serum IgG and robust mucosal IgA responses upon intranasal administration of the CCD/RBD-HR vaccine. This novel approach conferred complete protection against Omicron infection in animal models, with nearly undetectable viral RNA levels in the respiratory tract of immunized mice. Additionally, the vaccine facilitated the generation of tissue-resident memory T cells, underscoring its potential to provide long-lasting immunity. The study also identified the crucial role of nasal epithelial cells as antigen-presenting cells, further illuminating the mechanisms by which intranasal vaccination can enhance immune responses.
In summary, the findings emphasize the potential of intranasal CCD/RBD-HR vaccines as a promising platform for developing effective mucosal vaccines against COVID-19 and other respiratory pathogens, paving the way for future research and clinical applications in the realm of infectious disease prevention.
Reference
Lei, Hong, et al. “Cationic Crosslinked Carbon Dots-Adjuvanted Intranasal Vaccine Induces Protective Immunity Against Omicron-Included SARS-CoV-2 Variants.” Nature Communications, vol. 14, no. 2678, 2023, doi:10.1038/s41467-023-38066-8.
