Scientists develop cerasomes as stable lipid nanocarriers to enhance the delivery of paclitaxel across the blood-brain barrier for improved glioblastoma therapy.
Key Preview
Research Question
The study investigates the potential of cerasomes, a modified form of liposomes, to deliver therapeutic agents effectively across the blood-brain barrier (BBB) for glioma treatment.
Research Design and Strategy
The research employs two methods—thin film hydration and ethanol sol injection—to produce cerasomes with varying compositions, focusing on their stability, drug delivery capabilities, and efficacy in glioma therapy.
Method
Cerasomes were synthesized using surfactants to enhance stability. The evaluation included drug loading, cytotoxicity assessments, and in vivo studies on rat models to examine BBB penetration.
Key Results
Cerasomes loaded with paclitaxel demonstrated a 36-fold increase in cytotoxicity against T98G glioblastoma cells compared to free drug. The study confirmed effective delivery of fluorescent dye rhodamine B across the BBB in rats.
Significance of the Research
This work presents cerasomes as promising nanocarriers for enhancing drug delivery to the brain, potentially improving glioma treatment outcomes through increased drug efficacy and stability.
Introduction
Glioma, particularly glioblastoma multiforme (GBM), is one of the most aggressive and challenging forms of brain cancer. Characterized by rapid proliferation and invasive growth, GBM is known for its poor prognosis, with a median survival rate of only 15 months despite aggressive treatment. The complexity of gliomas arises from their heterogeneous nature and their location within the brain, which creates significant obstacles for effective treatment. Standard therapies for glioma typically involve a combination of surgical resection, radiation therapy, and chemotherapy. Among chemotherapeutic agents, temozolomide and paclitaxel have been used; however, their efficacy is often limited by challenges in achieving sufficient drug concentrations at the tumor site.
Traditional drug delivery strategies primarily rely on systemic administration, which can lead to suboptimal therapeutic concentrations at the tumor site. This approach often results in non-specific distribution of the drug, leading to severe side effects and limited efficacy against the tumor. Furthermore, the presence of the blood-brain barrier (BBB), a selective permeability barrier that protects the brain from harmful substances, poses a significant challenge. This barrier restricts the passage of many therapeutic agents, thus limiting their effectiveness in treating brain tumors. Consequently, the inability to deliver adequate drug concentrations directly to gliomas contributes to treatment resistance and disease recurrence.
To overcome these limitations, the development of innovative drug delivery systems has become a focal point in glioma research. One promising approach is the use of cerasomes, modified lipid nanoparticles that incorporate covalent siloxane networks for enhanced stability and drug encapsulation. Unlike traditional liposomes, cerasomes possess improved morphological stability and resistance to surfactant-induced solubilization, which makes them an attractive option for drug delivery. Their unique structure allows for controlled release of therapeutic agents and potential modification with targeting ligands, thereby enhancing their ability to cross the BBB and deliver drugs directly to tumor cells. By leveraging these advanced nanocarriers, there is significant potential to improve therapeutic outcomes for glioma patients, leading to more effective treatment strategies that can overcome the challenges of conventional drug delivery methods.
Research Team and Aim
The research team behind this study was led by Rais Pavlov, who conducted this research at the Arbuzov Institute of Organic and Physical Chemistry, part of the Russian Academy of Sciences. The study was published in 2023 in the International Journal of Molecular Sciences under the title “The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy.” The team comprised experts in various fields, including Elvira Romanova, Denis Kuznetsov, Anna Lyubina, Syumbelya Amerhanova, Alexandra Voloshina, Daina Buzyurova, Vasily Babaev, Irina Zueva, Konstantin Petrov, Svetlana Lukashenko, Gulnara Gaynanova, and Lucia Zakharova.
The aim of the research, as articulated by the lead researcher, was to “develop stable cerasomes for drug delivery to glioblastoma cells, ultimately enhancing the therapeutic effects of paclitaxel.” This objective reflects the team’s commitment to addressing the challenges associated with conventional drug delivery methods in glioma therapy, emphasizing the potential of cerasomes as innovative nanocarriers.
Experimental Process
Experiment 1: Cerasome Preparation via Ethanol Sol Injection Method
Primary Technique: The primary technique used in this research to prepare cerasomes was the ethanol sol injection method. This method was chosen for its ability to produce stable nanoparticles with precise size control and low polydispersity.
Key Steps:
- Stock solutions of cerasome-forming lipid (CFL16) were prepared in acidified ethanol at a concentration of 1 mg/mL.
- The solutions were incubated for a minimum of 12 hours at room temperature to allow complete hydrolysis of the triethoxysilyl groups.
- The CFL16 solution was then slowly injected into Milli-Q water under vigorous stirring to promote self-assembly into vesicular structures.
- The resulting cerasomes were extruded through a series of filters to achieve uniform particle size, specifically targeting a hydrodynamic diameter of approximately 210 nm.
Data Collection and Analysis: The size and polydispersity index (PdI) of the cerasomes were measured using dynamic light scattering (DLS). The zeta potential was also recorded to assess the stability of the nanoparticles.
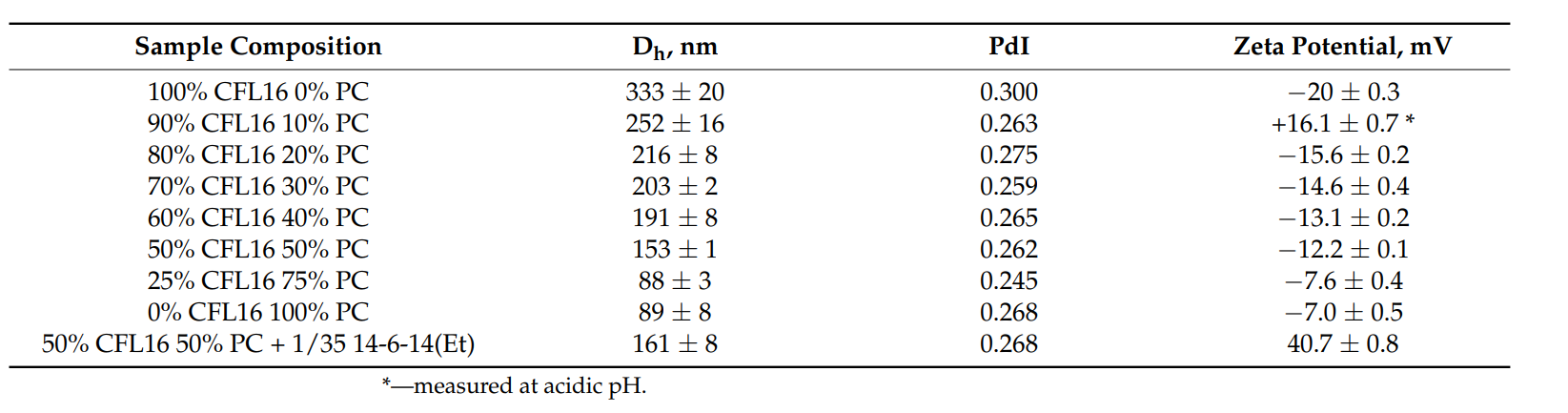 Table 1. DLS characteristics hybrid cerasomes prepared via thin film hydration with varied composition of lipid and the effect of modification with 14-6-14(Et) on the zeta potential at ph=7.4.
Table 1. DLS characteristics hybrid cerasomes prepared via thin film hydration with varied composition of lipid and the effect of modification with 14-6-14(Et) on the zeta potential at ph=7.4.
Result: The ethanol sol injection method yielded cerasomes with a hydrodynamic diameter of about 210 nm and a PdI of 0.1–0.15, indicating low size variability. The zeta potential measurement showed a value of approximately −45 mV, suggesting good stability of the nanoparticles.
Novel Aspect: This method of cerasome preparation represents an innovative approach to enhancing the morphological stability of lipid nanoparticles compared to traditional liposomes, which often suffer from aggregation and instability in physiological conditions.
Experiment 2: Cerasome Preparation via Thin Film Hydration Method
Primary Technique: The second method employed for cerasome preparation was the thin film hydration technique, which was evaluated for its effectiveness in producing stable formulations.
Key Steps:
- A mixture of CFL16 and phosphatidylcholine (PC) was dissolved in chloroform to form a lipid film.
- The solvent was evaporated under reduced pressure to create a thin lipid film on the walls of the flask.
- The lipid film was then hydrated with a mildly acidic aqueous solution (1 mM HCl) at pH 3 to allow for slow hydrolysis and subsequent formation of cerasomes.
- Tween 80 was added to the hydration mixture to enhance the ability of cerasomes to penetrate the blood-brain barrier (BBB).
- The mixture was vortexed and sonicated to achieve the desired nanoparticle size.
Data Collection and Analysis: The hydrodynamic diameter, PdI, and zeta potential were measured using DLS. Transmission electron microscopy (TEM) was utilized to visualize the morphology and confirm the vesicular structure of the cerasomes.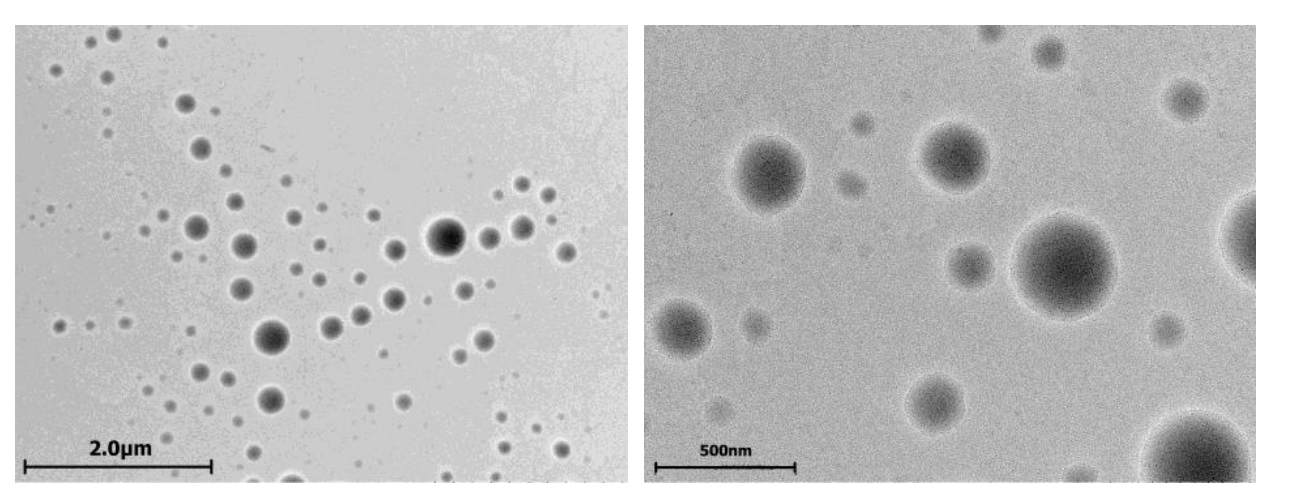
Figure 1. TEM images of 50% CFL16 50% PC cerasomes prepared via thin film. hydration method.
Result: The resulting cerasomes had hydrodynamic diameters ranging from 300 to 400 nm and demonstrated a zeta potential that shifted from positive in acidic conditions to approximately −10 to −20 mV upon dilution in physiological buffer, indicating successful formation of stable cerasomes.
Novel Aspect: The incorporation of Tween 80 into the thin film hydration method significantly improved the consistency and reliability of cerasome preparation, offering advantages over traditional methods that often yield less stable formulations.
Experiment 3: Cytotoxicity Assessment
Primary Technique: Cytotoxicity of the cerasomes was assessed using the MTT assay against the T98G glioblastoma cell line, a critical step in evaluating their therapeutic potential.
Key Steps:
- T98G cells were cultured in a 96-well plate and incubated until they formed a monolayer.
- Following this, cerasomes loaded with paclitaxel (PTX) and control formulations were added to the wells in various concentrations.
- After 24 hours of incubation, the media was replaced with MTT solution (0.5 mg/mL) and incubated for an additional 4 hours to allow for formazan crystal formation.
- The resulting formazan crystals were dissolved in DMSO, and the optical density was measured at 540 nm using a microplate reader.
Data Collection and Analysis: The IC50 values were calculated using the dose-response curves generated from the optical density readings. Statistical significance was determined using appropriate tests to compare the cytotoxicity of PTX-loaded cerasomes versus free PTX.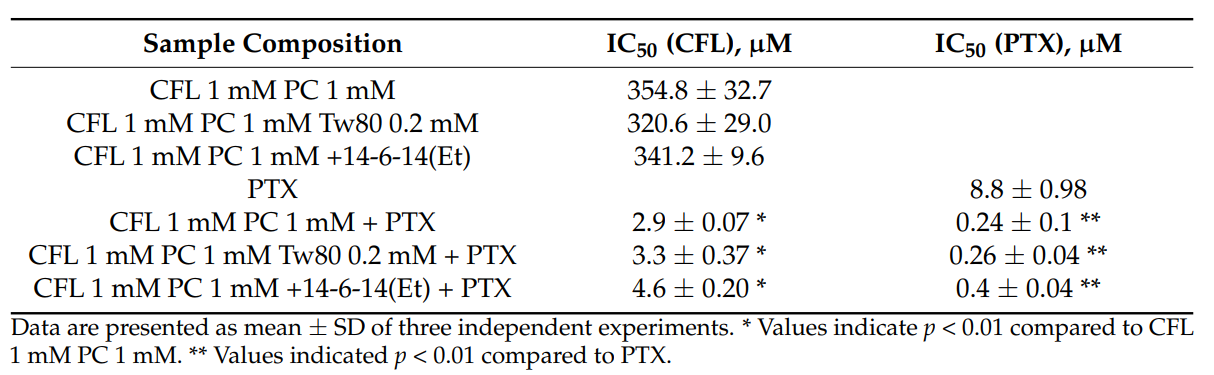
Cytotoxicity of hybrid cerasomes with different compositions, free PTX and PTX formulated in cerasomes toward T98G glioma cell line evaluated by 24 h MTT test.
Result: Cerasomes loaded with PTX demonstrated an IC50 of 2.9 µM, significantly lower than the IC50 of free PTX at 8.8 µM, indicating a 36-fold increase in cytotoxicity.
Novel Aspect: The enhanced cytotoxicity of PTX-loaded cerasomes underscores their potential as a novel drug delivery system, providing a substantial improvement over traditional formulations that often struggle with bioavailability and efficacy.
Experiment 4: In Vivo BBB Penetration Studies
Primary Technique: The ability of cerasomes to cross the blood-brain barrier (BBB) was evaluated through in vivo studies using a fluorescent dye (rhodamine B) to track cerasome distribution in rat brain tissue.
Key Steps:
- Wistar rats were intravenously injected with either free rhodamine B or rhodamine B-loaded cerasomes.
- After a two-hour post-injection period, the rats were anesthetized and perfused with cold phosphate-buffered saline to clear the blood circulation.
- Brain tissues were harvested and sectioned at 10 µm thickness using a cryotome.
- Confocal microscopy was performed to visualize and quantify the fluorescent signal of rhodamine B in brain slices.
Data Collection and Analysis: The intensity of fluorescence in brain tissue slices was analyzed using confocal imaging software to assess the distribution and penetration of cerasomes compared to free rhodamine B.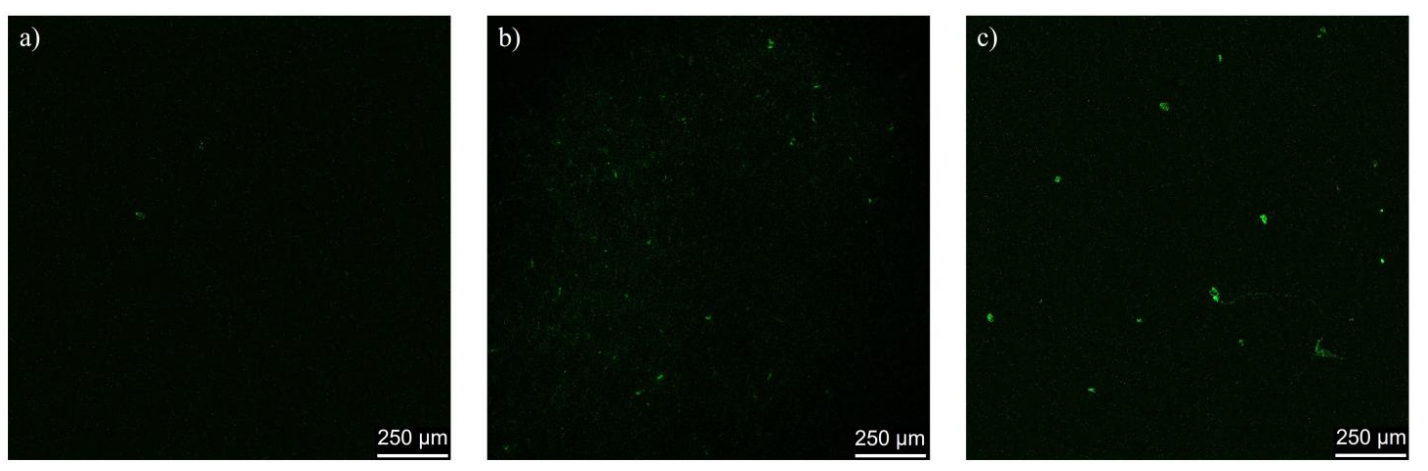
Figure 2. Rat brain slices after administration of (a) free rhodamine B; (b) plain rhodamine B-loaded cerasomes; © Tween 80 cerasomes labeled with rhodamine B
Result: The images indicated a significant increase in fluorescent intensity within the brain tissue of rats administered rhodamine B-loaded cerasomes compared to those that received free rhodamine B, confirming successful BBB penetration.
Novel Aspect: This study provides compelling evidence for the efficacy of cerasomes as a delivery vehicle for therapeutic agents across the BBB, representing a significant advancement over conventional drug delivery systems that typically fail to achieve effective brain targeting.
Conclusion
The successful development of this drug delivery system was achieved through the innovative design and synthesis of cerasomes, which demonstrated enhanced stability and efficacy in delivering therapeutic agents across the blood-brain barrier (BBB). By employing two distinct preparation methods—ethanol sol injection and thin film hydration—the researchers were able to produce cerasomes with optimal size, stability, and drug encapsulation capabilities. The incorporation of surfactants, such as Tween 80, further improved the cerasomes’ ability to penetrate the BBB, making them a promising candidate for glioma therapy.
A highlight of the study is the significant enhancement in the cytotoxicity of paclitaxel when loaded into cerasomes, achieving an impressive 36-fold increase in efficacy against T98G glioblastoma cells compared to free drug formulations. Additionally, the successful in vivo demonstration of cerasome-mediated delivery of a fluorescent dye across the BBB underscores their potential as effective nanocarriers for targeted drug delivery. Overall, this research presents cerasomes as a powerful and innovative approach to improving therapeutic outcomes for patients with glioma, paving the way for more effective treatment strategies in the future.
Reference
Pavlov, Rais, et al. “The Formation of Morphologically Stable Lipid Nanocarriers for Glioma Therapy.” International Journal of Molecular Sciences, vol. 24, no. 4, 2023, article 3632. https://doi.org/10.3390/ijms24043632.
