Editor: Nina
Scientists develop a cannabidiol-loaded starch nanoparticle system for enhanced nose-to-brain delivery and significant anti-inflammatory effects in neuroinflammatory models, offering a promising therapeutic strategy for neurodegenerative diseases.
Key Preview
- Research Question: This study investigates how to effectively deliver cannabidiol (CBD) to the brain to address neuroinflammation, a common pathology in various neurodegenerative diseases.
- Research Design and Strategy: The researchers developed a novel nanoparticulate system using crosslinked starch to facilitate the intranasal delivery of CBD, allowing for direct targeting to the central nervous system (CNS).
- Method: Cannabidiol-loaded starch nanoparticles (SNPs) were created via a nanoprecipitation method and characterized for their size, release profile, and anti-inflammatory activity in vitro using BV2 microglia cells.
- Key Results: Intranasal administration of CBD-loaded SNPs resulted in significantly higher CBD concentrations in the brain compared to a standard CBD solution. The nanoparticles showed effective anti-inflammatory properties, reducing nitric oxide (NO) production and interleukin-6 (IL-6) levels in LPS-induced inflamed cells.
- Significance of the Research: This research provides a promising platform for enhanced CBD delivery to the brain and potential treatments for neuroinflammatory conditions, showcasing the advantages of using nanoparticle technology in drug delivery.
Introduction
Neurodegenerative diseases, such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease, are characterized by progressive neuronal loss and accompanying neuroinflammation. These conditions significantly impair cognitive and motor functions, leading to a substantial decline in quality of life for affected individuals. The pathophysiology of neurodegenerative diseases often involves chronic inflammation within the central nervous system (CNS), primarily driven by the activation of microglia and the release of pro-inflammatory cytokines. Effective management of these diseases is critical, given the aging population and the increasing prevalence of such disorders worldwide.
Traditional treatment strategies for neurodegenerative diseases often rely on systemic drug administration, typically via oral or intravenous routes. While these methods can be effective in achieving therapeutic concentrations of medications, they frequently encounter significant challenges. One major issue is the blood-brain barrier (BBB), a selective permeability barrier that restricts the passage of therapeutic agents into the brain. As a result, many drugs exhibit low bioavailability and limited efficacy when administered systemically, necessitating higher doses that may exacerbate side effects. Furthermore, the first-pass metabolism encountered with oral administration can further diminish the therapeutic effects of drugs, leading to suboptimal treatment outcomes.
These challenges underline the pressing need for innovative drug delivery strategies that can enhance the targeting and efficacy of therapies for neurodegenerative diseases. One promising approach involves the use of nanoparticle-based systems to facilitate direct delivery of therapeutics to the brain. By employing techniques such as intranasal administration, nanoparticles can bypass the BBB and deliver drugs directly to the CNS, reducing systemic exposure and enhancing bioavailability. This innovative drug delivery strategy not only aims to improve the therapeutic index of drugs like cannabidiol (CBD) but also to mitigate the inflammatory processes associated with neurodegenerative diseases, thereby improving patient outcomes and quality of life.
Research Team and Aim
The research was conducted by a team led by Ilya Eydelman, alongside collaborators Na’ama Zehavi, Valeria Feinshtein, Dinesh Kumar, Shimon Ben-Shabat, and Amnon C. Sintov from Ben-Gurion University of the Negev, Israel. This study was conducted over the course of an academic year, culminating in the publication of their findings in the paper titled “Cannabidiol-Loaded Nanoparticles Based on Crosslinked Starch: Anti-Inflammatory Activity and Improved Nose-to-Brain Delivery,” which was published in the journal Pharmaceutics.
The aim of the research, as articulated by the lead researcher, was to develop and characterize a new nanoparticulate system that enhances CBD delivery to the brain while demonstrating anti-inflammatory effects in neuroinflammatory models. This innovative approach seeks to provide a foundation for future therapies targeting neurodegenerative diseases, leveraging the benefits of nanoparticle technology for effective drug delivery.
Experimental Process
Experiment 1: Preparation of Cannabidiol-Loaded Starch Nanoparticles (SNPs)
Primary Technique
The primary technique employed in this study is the nanoprecipitation method. This technique was chosen for its ability to create nanoparticles with controlled size and drug loading capacity through the rapid mixing of a polymer solution with a non-solvent.
Key Steps
- Preparation of Starch Slurry: Corn starch (1 g) was mixed with sodium hydroxide (0.8 g), urea (1 g), and double-distilled water (50 mL) until completely dissolved, resulting in a homogeneous slurry.
- Acidification: The starch slurry was then acidified to pH 3 using concentrated hydrochloric acid (32%).
- Crosslinking: An aliquot of the acidified starch slurry (20 mL) was diluted with purified water and mixed with divanillin (5 mg/mL).
- Nanoprecipitation: An ethanolic solution of cannabidiol (CBD; 2 mg or 5 mg in 15.4 mL) was dripped into the starch-divanillin mixture using a syringe pump at a rate of 0.2 mL/min while stirring constantly at 700 rpm in a heated bath (40 °C).
- Evaporation and Centrifugation: After nanoprecipitation, the mixture was stirred for an additional hour, and the remaining ethanol was evaporated using a rotary evaporator. The resulting nanoparticles were centrifuged at 250 rcf for 4 minutes, and the supernatant was collected.
Data Collection and Analysis
Data on the size and entrapment efficiency (EE) of the nanoparticles were collected using nanoparticle tracking analysis (NTA) and high-performance liquid chromatography (HPLC). The EE was calculated as a percentage of the total mass of CBD used in the formulation.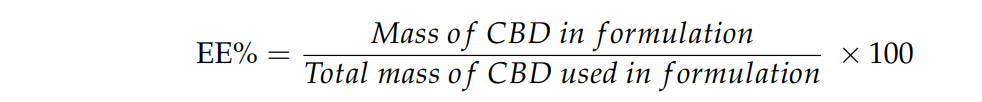
Figure 1. The entrapment efficiency percentage (EE%) was calculated according to the above equation.
Result
The average size of the SNPs was determined to be approximately 200 nm, with an EE ranging from 29% to 39% depending on the formulation conditions, particularly pH and crosslinker concentration.
Novel Aspect
This study introduces the use of crosslinked starch as a biodegradable polymer for nanoparticle formation, presenting an innovative alternative to traditional synthetic polymers. The approach enhances the stability and drug-loading capacity of the nanoparticles, potentially improving their therapeutic efficacy in CNS-targeted drug delivery.
Experiment 2: In Vitro Evaluation of Anti-Inflammatory Activity
Primary Technique
The in vitro evaluation of anti-inflammatory activity was conducted using BV2 microglia cell cultures. This model was selected due to its relevance in studying neuroinflammation associated with neurodegenerative diseases.
Key Steps
- Cell Culture Preparation: BV2 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics.
- Induction of Inflammation: Cells were pre-treated with lipopolysaccharides (LPS; 7 ng/mL) for 24 hours to induce an inflammatory response.
- Treatment with SNPs: Post LPS treatment, cells were treated with either CBD-loaded SNPs or unloaded SNPs for 24 hours.
- Assessment of Nitric Oxide (NO) Production: The medium was collected, and NO levels were quantified using the Griess reagent assay.
Data Collection and Analysis
Data on NO production were statistically analyzed using the Student’s t-test to compare treated groups against LPS control groups. Results were expressed as mean ± standard deviation (SD).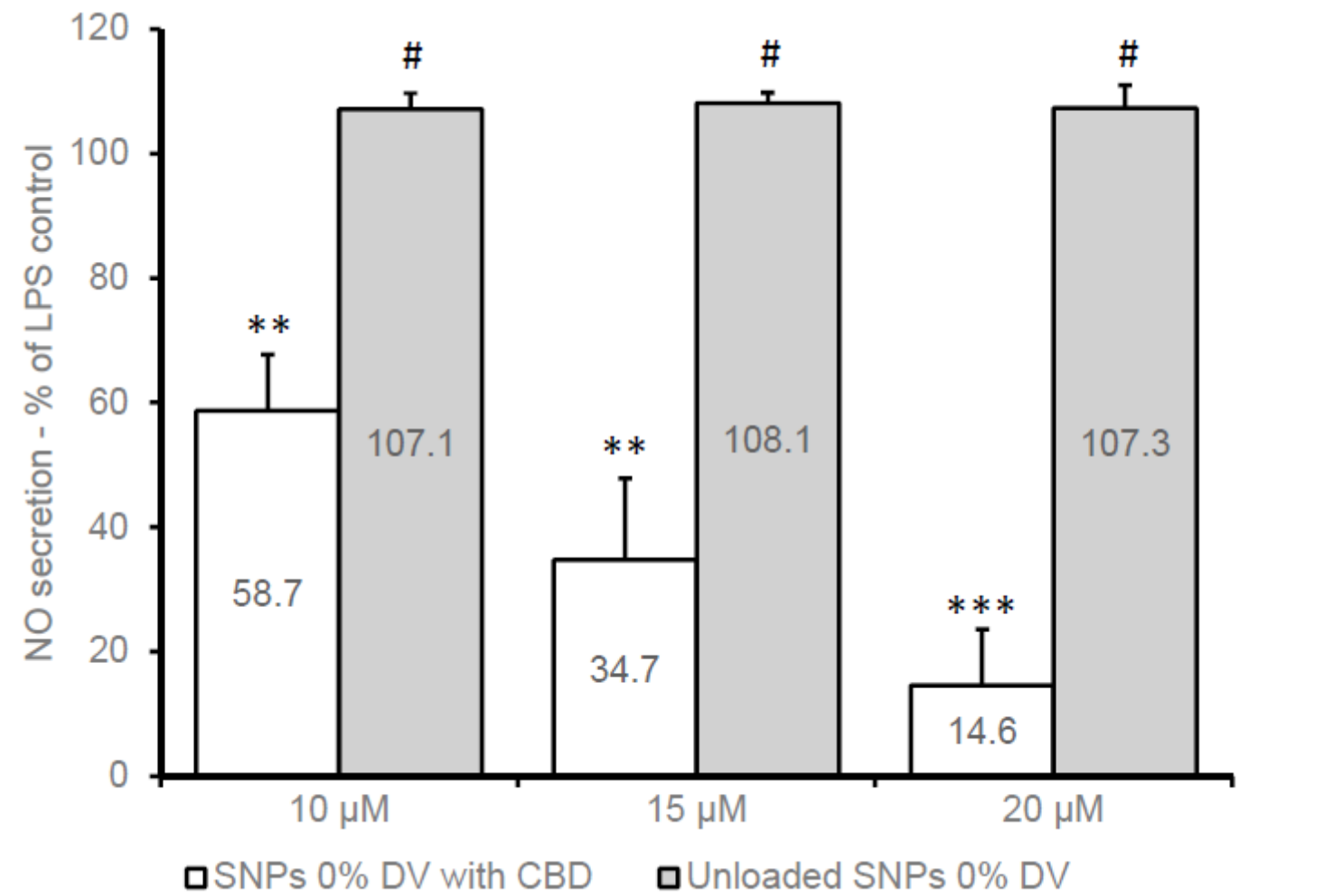
Figure 2. Nitric oxide secretion in BV2 cells 22 h after treatment with CBD-loaded and unloaded SNPs in medium with 1% FCS and LPS (7 ng/mL). Mean (±SD) of three independent experiments (n = 9). ** p < 0.005 against LPS control; *** p < 0.0002 against LPS control; # not significant against LPS control.
Result
CBD-loaded SNPs significantly reduced NO production in LPS-induced BV2 cells, demonstrating an anti-inflammatory effect comparable to glucocorticoids, with reductions of approximately 58.7% at higher concentrations.
Novel Aspect
The use of SNPs for CBD delivery not only enhances the bioavailability of CBD but also effectively reduces pro-inflammatory markers in a cellular model of neuroinflammation, showcasing a promising strategy for treating neurodegenerative diseases.
Experiment 3: Pharmacokinetic Study in Rats
Primary Technique
The pharmacokinetic study utilized the intranasal administration technique to evaluate the brain delivery of CBD-loaded SNPs compared to a conventional CBD solution.
Key Steps
- Animal Preparation: Male Sprague Dawley rats (200-300 g) were randomly assigned to receive either CBD-loaded SNPs or a CBD solution.
- Intranasal Administration: Each rat received a dose of 100 µg of CBD (20 µL per nostril).
- Euthanasia and Brain Collection: Following predetermined time points (5, 10, 15 minutes), animals were euthanized, and their brains were excised and frozen at -80 °C.
- Extraction and Analysis: Brain tissue was lyophilized, crushed, and extracted with methanol for CBD analysis via HPLC.
Data Collection and Analysis
The concentration of CBD in brain tissue was determined and quantified using HPLC. The peak brain concentration (Cmax) and time to peak concentration (tmax) were analyzed to assess the pharmacokinetic profiles.
Result
The Cmax for CBD-loaded SNPs was found to be 5.22 µg/g of brain tissue at 10 minutes post-administration, while the CBD solution did not yield detectable levels in the brain.
Novel Aspect
This study demonstrates that intranasal delivery of CBD-loaded SNPs results in significantly higher brain concentrations compared to conventional formulations. This novel route of administration effectively bypasses the first-pass metabolism, enhancing the potential for CNS-targeted therapies.
Experiment 4: Cellular Uptake of Nanoparticles
Primary Technique
The cellular uptake assessment utilized fluorescence imaging and quantitative analysis to determine the internalization of CBD-loaded SNPs in BV2 cells.
Key Steps
- Cell Preparation: BV2 cells were seeded in 6-well plates and incubated overnight.
- Treatment with SNPs: Cells were treated with CBD-loaded SNPs for 24 hours.
- Cell Harvesting: Post-treatment, cells were washed, detached, and divided into fractions for analysis.
- Extraction: Cells were treated with PBS, Triton X-100, and methanol to recover CBD from cell components.
Data Collection and Analysis
The amount of CBD in each fraction was analyzed using HPLC. The data were presented as total CBD recovered and percentage of administered CBD found in cells.
Result
The internalized CBD was quantified, revealing that approximately 1.4% of administered CBD was found within cells, indicating effective uptake of the nanoparticles.
Novel Aspect
This experiment highlights the ability of SNPs to facilitate cellular uptake of CBD, demonstrating an innovative approach to enhance drug delivery to targeted cells, particularly within the CNS.
Conclusion
The successful development of the cannabidiol (CBD)-loaded starch nanoparticle (SNP) delivery system has been achieved through a series of meticulous experimental processes that focused on optimizing nanoparticle formulation, enhancing drug entrapment, and evaluating therapeutic efficacy. By employing a nanoprecipitation method with crosslinked starch, the researchers were able to create a stable and biodegradable carrier for CBD that effectively targets the central nervous system (CNS). The innovative use of intranasal administration allows for direct delivery to the brain, bypassing the blood-brain barrier and significantly improving bioavailability compared to traditional oral or intravenous routes.
The highlights of this study include the demonstration that intranasal administration of CBD-loaded SNPs leads to significantly higher concentrations of CBD in the brain, with a peak concentration of 5.22 µg/g tissue observed within 10 minutes post-administration. Additionally, the SNPs exhibited potent anti-inflammatory effects, significantly reducing nitric oxide production and interleukin-6 levels in LPS-induced BV2 microglia cells, thereby underscoring their potential as a therapeutic strategy for neuroinflammatory conditions. Overall, this research provides a promising platform for enhancing CBD delivery to the brain, paving the way for improved treatment options for patients suffering from neurodegenerative diseases.
Reference
Eydelman, Ilya, et al. “Cannabidiol-Loaded Nanoparticles Based on Crosslinked Starch: Anti-Inflammatory Activity and Improved Nose-to-Brain Delivery.” Pharmaceutics, vol. 15, no. 7, 2023, article 1803. MDPI, https://doi.org/10.3390/pharmaceutics15071803.
