Editor: Nina
Scientists develop a copper-induced injectable hydrogel enriched with nitric oxide and PD-L1 antibodies to enhance immunotherapy by amplifying immunogenic cell death and regulating cancer-associated fibroblasts for improved anti-tumor efficacy.
Key Preview
Research Question
The study investigates how a copper-induced injectable hydrogel, enriched with nitric oxide (NO) and programmed death-ligand 1 (PD-L1) antibodies, can enhance immunotherapy by amplifying immunogenic cell death (ICD) and regulating cancer-associated fibroblasts (CAFs). Specifically, the research seeks to address the challenges posed by the immunosuppressive tumor microenvironment (TME) and the inefficacy of current immunotherapy approaches.
Research Design and Strategy
The research employs a combination of in vitro and in vivo methodologies to evaluate the therapeutic efficacy of the injectable hydrogel. The study incorporates various assays, including flow cytometry, enzyme-linked immunosorbent assays (ELISA), and transcriptome analyses, to explore the immunological and anti-tumor responses elicited by the hydrogel in cancer models.
Method
The researchers designed a hydrogel that combines the photothermal effects of copper ions with the release of NO and encapsulated PD-L1 antibodies. The therapeutic effects were evaluated using 4T1 cancer cells and CAFs in vitro, as well as in 4T1 tumor-bearing mice in vivo.
Key Results
The study demonstrated that the injectable hydrogel significantly amplified ICD, leading to an increased infiltration of immune cells, reduced CAF levels, and a decrease in M2 macrophage proportions. Specifically, the hydrogel treatment resulted in a 76.9% inhibition of tumor growth in vivo, offering a promising new avenue for enhancing the efficacy of cancer immunotherapy.
Significance of the Research
This research provides critical insights into the role of CAFs in the TME and their impact on immunotherapy outcomes. The findings highlight the potential of utilizing a copper-induced hydrogel to overcome immunosuppressive barriers, thus advancing clinical approaches to cancer treatment.
Introduction
Cancer remains one of the most significant global health challenges, characterized by uncontrolled cell growth and the ability to invade other tissues. It encompasses a wide range of diseases affecting various organs and systems, leading to considerable morbidity and mortality. Despite advances in surgical techniques, radiation therapy, and chemotherapy, cancer treatment often faces limitations, including the potential for resistance, recurrence, and adverse side effects. The complexity of the tumor microenvironment (TME), which is composed of not only tumor cells but also stromal cells, immune cells, and extracellular matrix components, further complicates effective treatment strategies.
Traditional drug delivery methods in cancer therapy typically involve systemic administration of chemotherapeutic agents. These agents are designed to target rapidly dividing cancer cells; however, they often lack specificity, leading to damage to healthy tissues and significant side effects. Furthermore, the heterogeneity of tumors can result in variable drug distribution and bioavailability, limiting the overall therapeutic efficacy. Consequently, patients frequently experience inadequate responses to treatment, leading to disease progression and diminished quality of life.
The challenges associated with conventional drug delivery approaches highlight the urgent need for innovative strategies that enhance the targeted delivery of therapeutics while minimizing systemic toxicity. One significant issue is the immunosuppressive nature of the TME, which can impede the infiltration and activation of immune cells necessary for an effective anti-tumor response. Additionally, the presence of cancer-associated fibroblasts (CAFs) in the TME contributes to drug resistance and immune evasion, further complicating treatment outcomes.
To address these challenges, the current study introduces an innovative drug delivery strategy utilizing a copper-induced injectable hydrogel that encapsulates nitric oxide (NO) donors and PD-L1 antibodies. This hydrogel is designed to enhance immunotherapy by promoting immunogenic cell death (ICD) and modulating the TME. By leveraging the photothermal properties of copper ions, the hydrogel not only facilitates localized drug release but also utilizes the tumor’s acidic microenvironment to activate the release of NO, which plays a critical role in depleting CAFs and reducing immunosuppressive signals. This multifaceted approach aims to overcome the limitations of traditional therapies and enhance the overall efficacy of cancer treatment.
Research Team and Aim
The research team for this study was led by Dr. Shuilin Shen, a prominent researcher at the School of Pharmaceutical Science, Nanjing Tech University. This research was conducted in 2023 and culminated in the publication of the paper titled “Copper-induced injectable hydrogel with nitric oxide for enhanced immunotherapy by amplifying immunogenic cell death and regulating cancer-associated fibroblasts.” This work was published in the journal Biomaterials Research.
The aim of the research, as articulated by Dr. Shen, is to explore the therapeutic potential of a copper-induced injectable hydrogel enriched with nitric oxide (NO) and programmed death-ligand 1 (PD-L1) antibodies. The study specifically seeks to enhance immunotherapy by amplifying immunogenic cell death (ICD) and regulating cancer-associated fibroblasts (CAFs) in order to improve treatment outcomes in cancer patients.
Experimental Process
Primary Technique
The primary technique utilized in this study is the development and characterization of a copper-induced injectable hydrogel. This hydrogel is designed to encapsulate nitric oxide (NO) donor and programmed death-ligand 1 (PD-L1) antibodies, aiming to enhance immunotherapy by amplifying immunogenic cell death (ICD) and modulating the tumor microenvironment (TME). The study integrates methodologies from materials science, cellular biology, and immunology to achieve its objectives.
Experiment 1: Hydrogel Preparation
Key Steps:
- Synthesize mercaptohyaluronic acid (HA-SH) by activating HA with EDC·HCl and NHS, followed by the addition of cystamine dihydrochloride to incorporate thiol groups.
- Conduct dialysis to purify HA-SH from unreacted materials.
- Mix HA-SH with copper ions (CuCl2) to induce the formation of a stable hydrogel via S-Cu coordination.
Data Collection and Analysis:
Characterization of the hydrogel structure was performed using scanning electron microscopy (SEM) to assess the morphology and porosity. Rheological measurements were taken using a dynamic shear rheometer to evaluate the mechanical properties of the hydrogel.
Figure 1. Photographs of the sol-to-gel transition and SEM image of the surface of the hydrogel.
Result:
The synthesized hydrogel demonstrated a stable three-dimensional network structure, confirmed by SEM imaging. Rheological testing revealed consistent storage (G’) and loss (G’’) moduli, indicating mechanical stability suitable for in vivo applications.
Novel Aspect:
The introduction of copper ions as both a cross-linker and photothermal agent distinguishes this hydrogel from traditional drug delivery systems, providing enhanced mechanical properties and localized heat generation.
Experiment 2: Photothermal Effect Evaluation
Key Steps:
- Prepare hydrogel samples with CuCl2 and subject them to near-infrared (NIR) laser irradiation at a power density of 0.8 W/cm² for 140 seconds.
- Measure the temperature changes in the hydrogel samples during irradiation using a K-type thermometer.
Data Collection and Analysis:
Temperature recordings were collected continuously during the laser exposure, with comparisons made between hydrogel samples and control groups (HA-SH and CuCl2 solutions without hydrogel).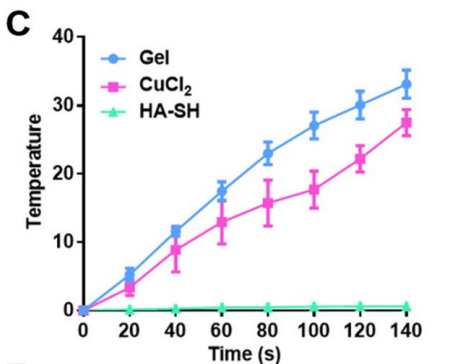
Figure 2. Temperature variation curve of hydrogel, C uCl 2 solution and HA-SH solution with NIR laser irradiation
Result:
The hydrogel exhibited a significant temperature increase, reaching approximately 45°C, indicative of effective photothermal conversion, while control groups showed minimal temperature fluctuation.
Novel Aspect:
This hydrogel’s capability to generate heat upon NIR irradiation represents a significant advancement over conventional systems, which often lack the ability to induce localized hyperthermia, enhancing its therapeutic potential.
Experiment 3: NO Release and Cytotoxicity Assessment
Key Steps:
- Encapsulate S-Nitrosoglutathione (GSNO) within the hydrogel and expose the samples to GSH (10 mM) to stimulate NO release.
- Conduct cytotoxicity assays on 4T1 cancer cells alongside the hydrogel treatments, both with and without NIR irradiation.
Data Collection and Analysis:
NO release was quantified using a Griess assay, while cell viability was assessed using the MTT assay, enabling determination of cytotoxic effects across varying concentrations of GSNO and CuCl2.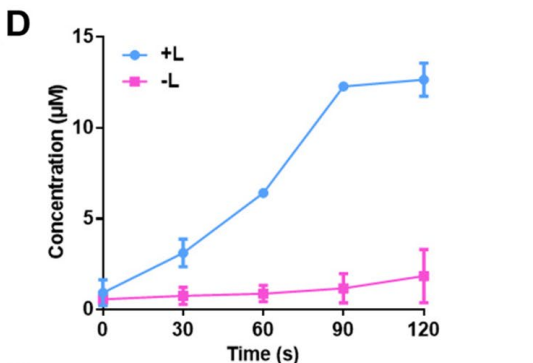
Figure 3. Release profles of NO from hydrogel with or without NIR laser irradiation.
Result:
The hydrogel facilitated a rapid NO release of approximately 140 μM within 3 hours upon GSH exposure. Cytotoxicity results indicated a 30% viability in 4T1 cells treated with G@Gel compared to controls, demonstrating enhanced cancer cell apoptosis due to NO and hyperthermia.
Novel Aspect:
The synergistic effect of NO release combined with photothermal therapy represents a novel dual-action approach, surpassing traditional single-modality treatments in efficacy against tumor cells.
Experiment 4: In Vivo Anti-Tumor Studies
Key Steps:
- Subcutaneously inject 4T1 tumor-bearing mice with various hydrogel formulations (G@Gel, aPD-L1@Gel, G/aP@Gel) and irradiate with NIR laser.
- Monitor tumor growth over a 21-day period, recording tumor volumes and weights.
Data Collection and Analysis:
Tumor sizes were calculated using the formula (width² × length × 0.5), and statistical analysis was conducted using one-way ANOVA to assess treatment efficacy across different groups.
Result:
The G/aP@Gel treated group exhibited a remarkable 76.9% reduction in tumor growth, contrasting sharply with control and other treatment groups, which showed limited efficacy.
Novel Aspect:
This study establishes a new combination strategy that integrates immunotherapy with localized photothermal and chemodynamic therapies, significantly enhancing treatment outcomes compared to traditional methods.
Experiment 5: Immune Response Evaluation
Key Steps:
- Collect tumor and lymph node tissues from treated mice post-experiment for flow cytometry analysis.
- Stain isolated immune cells with specific antibodies (CD3, CD8, CD11c, etc.) to assess T cell infiltration and dendritic cell maturation.
Data Collection and Analysis:
Flow cytometry was employed to quantify the percentage of immune cell populations, with results statistically analyzed to evaluate differences between treatment groups.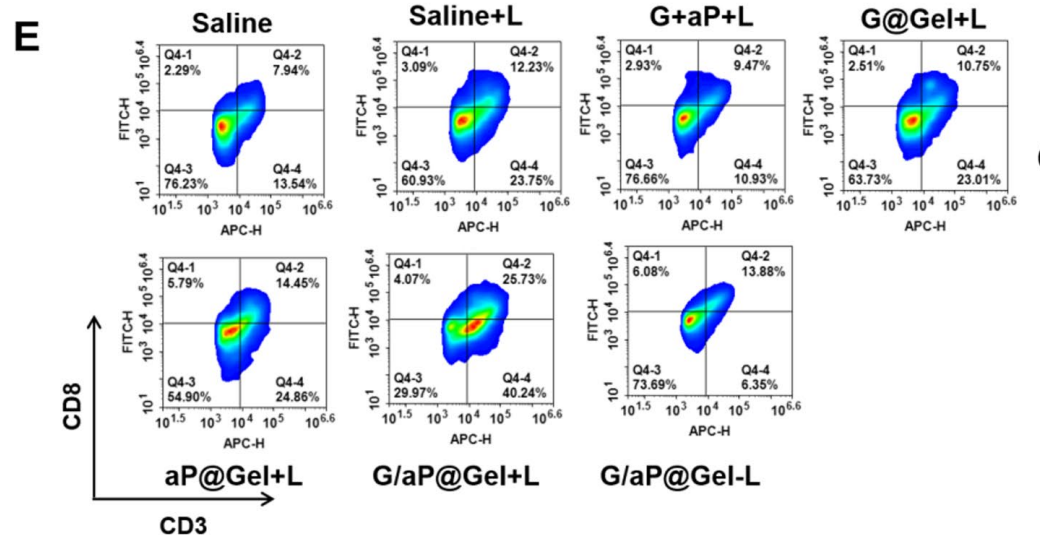
Figure 4. Flow cytometric examination of the intratumor infltration of C D8+T cells after various treatments
Result:
The G/aP@Gel with laser group showed a significant increase in CD8+ T cell infiltration and mature dendritic cells, supporting the hypothesis that the hydrogel effectively activates anti-tumor immunity.
Novel Aspect:
The ability of the hydrogel to enhance both innate and adaptive immune responses marks a departure from existing therapies, which often fail to adequately stimulate immune systems, thereby paving the way for more effective immunotherapeutic strategies.
Conclusion
The successful development of the copper-induced injectable hydrogel as an innovative drug delivery system was achieved through a strategic integration of advanced materials and therapeutic modalities. By leveraging the unique properties of copper ions, the research team created a hydrogel that not only serves as a carrier for nitric oxide (NO) donors and PD-L1 antibodies but also exhibits photothermal properties. This dual functionality facilitates localized drug release while simultaneously promoting hyperthermia, which enhances the therapeutic effect on cancer cells. The hydrogel’s design allows it to respond to the acidic tumor microenvironment, thereby ensuring efficient release of NO and effective modulation of the tumor microenvironment.
A significant highlight of the study is the demonstration that the injectable hydrogel markedly amplifies immunogenic cell death (ICD), resulting in increased immune cell infiltration and a notable reduction in cancer-associated fibroblasts (CAFs). Specifically, the hydrogel treatment led to an impressive 76.9% inhibition of tumor growth in vivo, showcasing its potential as a powerful tool to enhance the efficacy of cancer immunotherapy. These findings underscore the innovative nature of this drug delivery system and its capacity to address critical challenges in cancer treatment, paving the way for improved therapeutic outcomes for patients.
Reference
Shen, Shuilin, et al. “Copper-induced injectable hydrogel with nitric oxide for enhanced immunotherapy by amplifying immunogenic cell death and regulating cancer-associated fibroblasts.” Biomaterials Research, vol. 27, no. 44, 2023, https://doi.org/10.1186/s40824-023-00389-4.
