Editor: Nina
Scientists develop a multifunctional nanocomposite hydrogel incorporating BMP-2 and silver-embedded mesoporous silica nanoparticles to enhance bone regeneration while providing controlled release and antibacterial properties.
Key Preview
Research Question:
The study investigates how a multifunctional nanocomposite hydrogel can enhance bone regeneration, addressing the limitations of current bone grafting techniques, such as donor shortages and postoperative infections.
Research Design and Strategy:
The researchers designed a hydrogel that combines bone morphogenetic protein-2 (BMP-2) with silver (Ag+) core-embedded mesoporous silica nanoparticles (Ag@MSN), aiming to create a scaffold that promotes bone healing while providing antibacterial properties.
Method:
The method involved synthesizing the nanocomposite hydrogel by covalently bonding Ag@MSN to BMP-2, encapsulating this complex within silk fibroin methacryloyl (SilMA), and photo-crosslinking to achieve a stable structure.
Key Results:
The Ag@MSN-BMP-2/SilMA hydrogel showed significant improvements in bone regeneration in a rat skull defect model, with a bone mineral density of 0.21 g/cm³ after 8 weeks. Additionally, the hydrogel demonstrated sustained release of BMP-2 and effective antibacterial activity against E. coli and S. aureus.
Significance of the Research:
This research contributes to the field of tissue engineering by providing a novel biomaterial that not only supports osteogenesis but also mitigates infection risks, thereby enhancing the efficacy of bone regeneration strategies.
Introduction
Bone defects resulting from trauma, tumors, infections, or degenerative diseases significantly impact patients’ health and quality of life. These defects can lead to complications such as impaired mobility and chronic pain, and in severe cases, they may necessitate amputation. The traditional gold standard for treating bone defects has been the use of autologous and allogeneic bone grafts. These grafts, while effective, require surgical procedures to harvest tissue, which can lead to additional complications such as donor site morbidity, infection, and limited availability of graft material.
Current drug delivery strategies in traditional treatments often involve systemic administration of therapeutic agents, including growth factors like bone morphogenetic proteins (BMPs), which promote bone healing and regeneration. However, this approach presents significant challenges, including the rapid degradation and clearance of these biomolecules from the body, resulting in suboptimal therapeutic concentrations at the target site. Moreover, high doses of growth factors can lead to adverse effects, such as ectopic bone formation and immunological reactions, further complicating treatment outcomes.
In response to these challenges, innovative drug delivery strategies have emerged, focusing on the development of advanced biomaterials that can provide localized and sustained release of therapeutic agents. One such strategy involves the use of multifunctional nanocomposite hydrogels that encapsulate drugs while offering structural support to facilitate bone regeneration. These hydrogels not only protect and stabilize the drugs from degradation but also provide a favorable microenvironment for cellular activities, thereby enhancing the efficacy of treatment. By integrating antibacterial properties and osteogenic factors into a single delivery system, these innovative materials aim to address the multifaceted challenges associated with traditional bone regeneration therapies, paving the way for more effective and safer treatment options for patients with bone defects.
Research Team and Aim
The research was conducted by a collaborative team led by Dr. Yingji Mao, who played a crucial role in spearheading the investigation into innovative drug delivery strategies for bone regeneration. The study took place in 2023 and involved researchers from various institutions, primarily affiliated with the First Affiliated Hospital of Bengbu Medical College in China. The findings of this research were published in the article titled “A multifunctional nanocomposite hydrogel with controllable release behavior enhances bone regeneration” in the journal Regenerative Biomaterials.
The aim of the research, as articulated by Dr. Mao, was to “develop a hydrogel that enhances bone regeneration while providing a controlled release of osteogenic factors and antibacterial properties,” thereby addressing the limitations of existing bone grafting techniques and improving clinical outcomes for patients suffering from bone defects.
Experimental Process
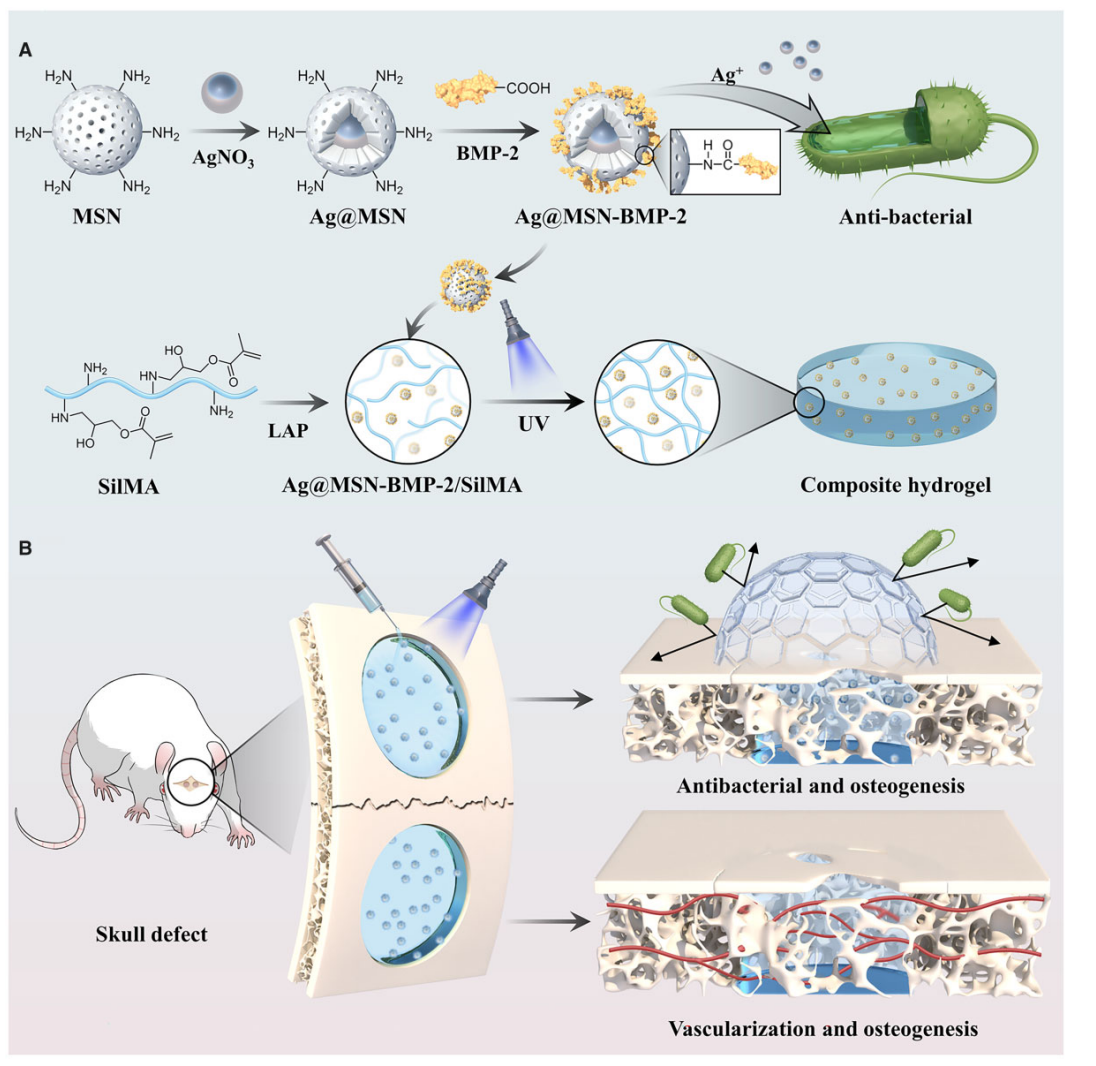
Figure 1. Schematic diagram of Ag@MSN-BMP-2/SilMA hydrogel to promote healing of tissue defects.
Experiment 1: Synthesis of Ag@MSN and Ag@MSN-BMP-2
Primary Technique:
The primary technique employed in this study involves the synthesis of mesoporous silica nanoparticles (MSNs) embedded with silver ions (Ag@MSN) and the subsequent covalent attachment of bone morphogenetic protein-2 (BMP-2) to these nanoparticles. This method establishes a multifunctional scaffold for bone regeneration applications.
Key Steps:
- Synthesis of MSNs: MSNs were prepared via a sol-gel process, using tetraethyl orthosilicate (TEOS) as the silica source. The reaction conditions were optimized to achieve a uniform particle size of approximately 120 nm.
- Embedding Silver Ions: A solution containing AgNO3 was added to the prepared MSNs, allowing Ag+ ions to be incorporated into the silica structure through a reduction process. The resultant Ag@MSN particles had an average diameter of 85 nm with a core-shell structure.
- Covalent Binding of BMP-2: BMP-2 was covalently attached to the surface of Ag@MSN using a crosslinking agent (EDC/NHS) in a sterile phosphate-buffered saline (PBS) solution. The reaction was stirred at room temperature for 24 hours and was followed by extensive washing to remove unbound peptides.
Data Collection and Analysis:
The successful synthesis of Ag@MSN and Ag@MSN-BMP-2 was confirmed using transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR). The loading capacity of BMP-2 onto Ag@MSN was calculated based on the initial and final concentrations of BMP-2, yielding an average loading rate of 14.9%.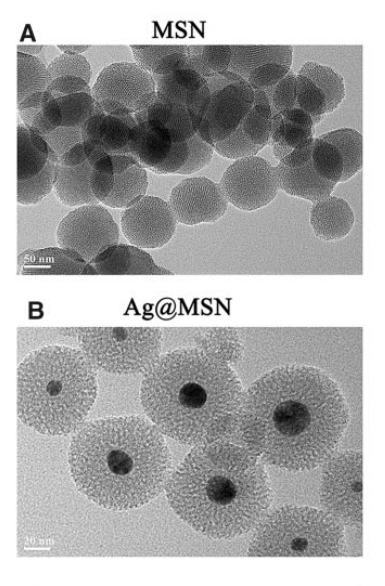
Figure 2. TEM images of MSN and Ag@MSN
Result:
The synthesized Ag@MSN-BMP-2 exhibited a well-defined core-shell structure with significant loading of BMP-2, confirming the efficacy of the synthesis method. The nanoparticles demonstrated a controlled release profile for BMP-2 over 28 days.
Novel Aspect:
This experiment innovatively combines BMP-2 with Ag@MSN, enhancing the osteogenic properties of the hydrogel while simultaneously providing antibacterial capabilities. This represents a significant improvement over traditional nano-delivery systems, which often lack multifunctionality.
Experiment 2: Preparation of SilMA Hydrogel
Primary Technique:
The primary technique for this experiment involved the preparation of silk fibroin methacryloyl (SilMA) hydrogels through photo-crosslinking. This approach allows the formation of a three-dimensional (3D) hydrogel network that serves as a scaffold for bone tissue engineering.
Key Steps:
- Dissolving Silk Fibroin: Silk fibroin was dissolved in a methacryloyl solution at 40-50°C to achieve a homogeneous blend.
- Photo-Crosslinking: Lithium phenyl-2,4,6-trimethylbenzoyl phosphinate (LAP) was mixed into the solution at a concentration of 0.5 g per 20 ml of PBS. The mixture was exposed to UV light for 30 seconds, initiating the crosslinking process that resulted in the formation of SilMA hydrogel.
- Sterilization: The resulting hydrogel was sterilized using a 0.22 µm sterile needle filter to ensure biocompatibility for subsequent biological assays.
Data Collection and Analysis:
The morphology of the SilMA hydrogel was assessed using scanning electron microscopy (SEM). Mechanical properties were evaluated through Young’s modulus testing, and the swelling ratio was determined by measuring the weight of the hydrogel before and after soaking in PBS.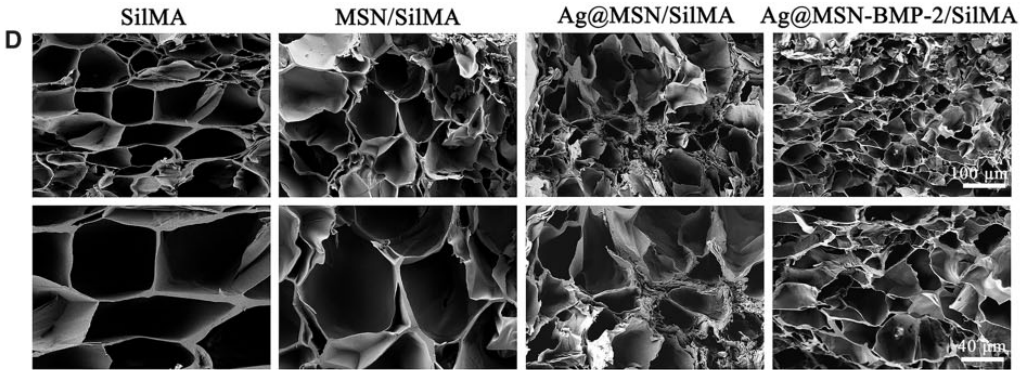
Figure 3. SEM images of the inner porous structures of SilMA, MSN/SilMA, Ag@MSN/SilMA and Ag@MSN-BMP-2/SilMA.
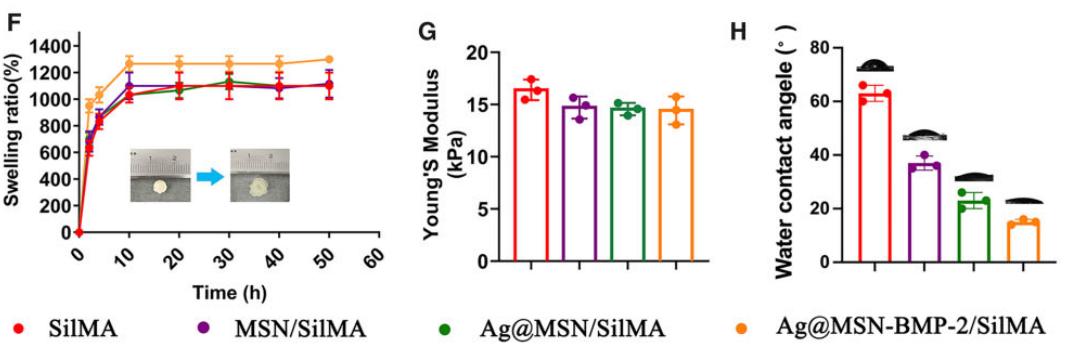
Figure 4. (F) Equilibrium swelling property of freeze-dried composite hydrogels. (G) Young’s modulus and (H) WCA of nanocomposite hydrogels.
Result:
The SilMA hydrogel demonstrated a Young’s modulus of approximately 16.83 kPa, suitable for supporting cell growth and differentiation. The swelling ratio exceeded 1200 wt% after 10 hours, indicating excellent hydration properties.
Novel Aspect:
The SilMA hydrogel represents a novel scaffold material that mimics the extracellular matrix, providing an advantageous environment for cellular activities compared to traditional hydrogels, which often lack structural integrity and biocompatibility.
Experiment 3: In Vitro Cell Viability and Osteogenic Differentiation Assays
Primary Technique:
The primary technique utilized in this experiment was cell viability and differentiation assays using bone marrow-derived mesenchymal stem cells (BMSCs) cultured on the Ag@MSN-BMP-2/SilMA hydrogels. This method assesses the biological performance of the developed scaffold in promoting cell survival and osteogenic differentiation.
Key Steps:
- Cell Culture: BMSCs were isolated and cultured in DMEM/F12 medium supplemented with 10% serum. The cells were seeded onto the hydrogel scaffolds at a density of 5 × 10^4 cells/ml.
- Live/Dead Staining: After 1, 4, and 7 days of culture, live/dead staining was performed using a calcein AM/PI kit to visualize cell viability under a confocal laser scanning microscope (CLSM).
- Osteogenic Induction: The cells were induced with osteogenic media containing 100 ng/ml BMP-2 and incubated for up to 14 days. Alkaline phosphatase (ALP) activity and mineralization were assessed using ALP staining and Alizarin Red S staining.
Data Collection and Analysis:
Cell viability was quantified using optical density measurements from the CCK-8 assay. ALP activity was statistically analyzed to determine the osteogenic differentiation capacity, and mineralization was visually assessed through staining quantification.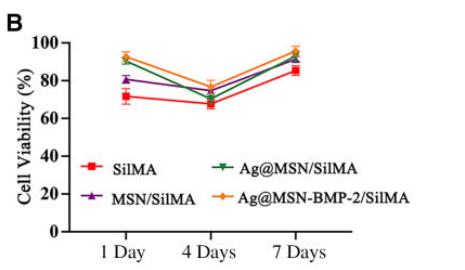
Figure 5. (B) Cell viability of BMSC cultures on different multifunctional nanocomposite hydrogels after 1, 4 and 7 days of culture.
Result:
BMSCs exhibited over 80% viability across all hydrogel groups, with the Ag@MSN-BMP-2/SilMA group displaying significantly higher ALP activity and mineralized nodule formation compared to control groups.
Novel Aspect:
This experiment showcases the dual functionality of the Ag@MSN-BMP-2/SilMA scaffold in promoting both cell viability and osteogenic differentiation, surpassing traditional single-function scaffolds that do not support simultaneous antimicrobial activity.
Experiment 4: In Vivo Bone Regeneration and Angiogenesis Assessment
Primary Technique:
The primary technique for this experiment was the evaluation of in vivo bone regeneration and angiogenesis using a rat skull defect model. This approach allowed for the assessment of the functional performance of the developed hydrogels in a biological context.
Key Steps:
- Surgical Procedure: Male SD rats were anesthetized, and a 10 mm skull defect was created using a circular drill. The Ag@MSN-BMP-2/SilMA hydrogel was then injected into the defect site and crosslinked in situ using UV light.
- Postoperative Care: Rats received intramuscular penicillin for 3 days post-surgery to prevent infection. They were monitored for signs of healing and recovery.
- Micro-CT and Histological Analysis: After 4 and 8 weeks, cranial samples were harvested for micro-computed tomography (micro-CT) analysis to assess bone mineral density (BMD) and new bone volume. Histological sections were stained with H&E and Masson’s trichrome to evaluate bone formation and vascularization.
Data Collection and Analysis:
Micro-CT data were quantitatively analyzed to assess BMD and bone volume/total volume (BV/TV) ratios. Histological sections were examined for new bone formation, and statistical significance was evaluated using appropriate software.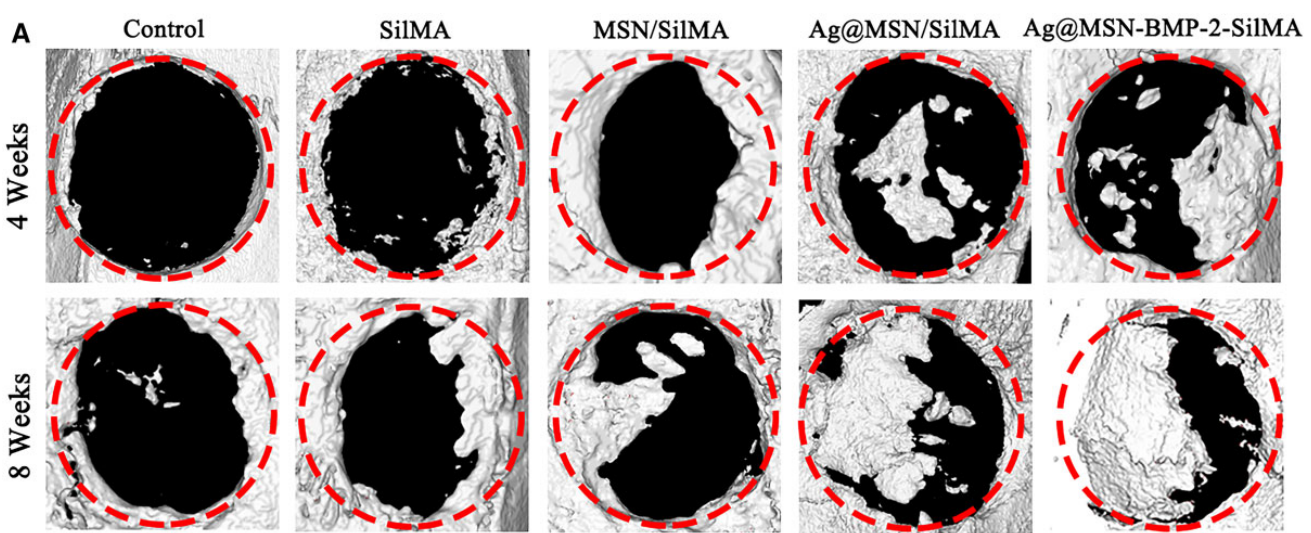
Figure 6. (A) Representative 3D reconstructed images of the skull defect after micro-CT scanning.
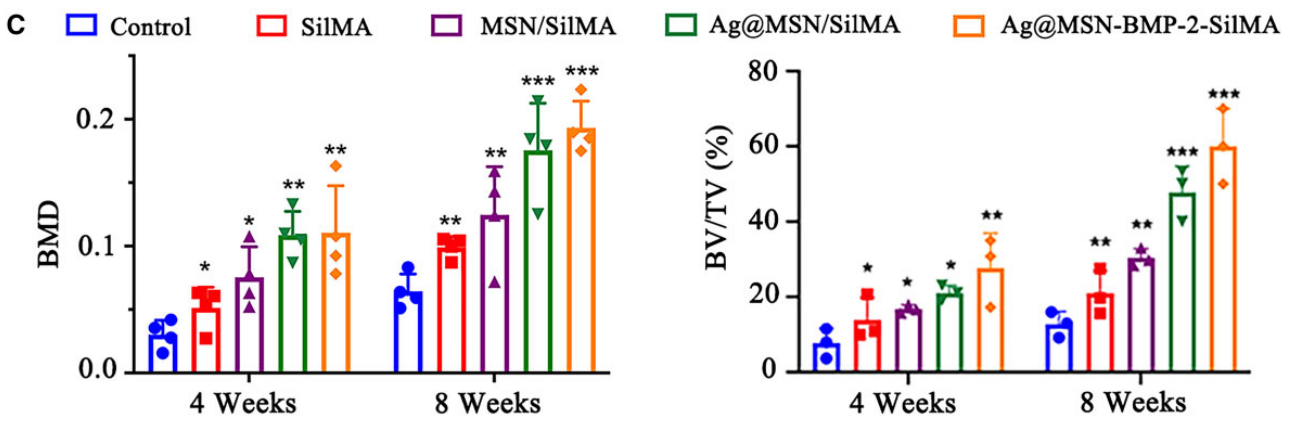
Figure 7. © BMD and BV/TV in the defect region (*P < 0.05, **P < 0.01 and ***P < 0.001, compared with the control group).
Result:
The Ag@MSN-BMP-2/SilMA group demonstrated significant improvements in BMD (0.21 g/cm³) and BV/TV (58.7%) compared to control groups, indicating effective bone regeneration and enhanced vascularization within the defect area.
Novel Aspect:
This in vivo study highlights the scaffold’s ability to promote not only bone regeneration but also angiogenesis, an essential factor for successful tissue engineering. The dual capability of the Ag@MSN-BMP-2/SilMA hydrogel to support both osteogenesis and vascularization represents a substantial advancement over conventional grafting techniques that do not address infection control or vascular needs.
Conclusion
The successful development of the Ag@MSN-BMP-2/SilMA hydrogel drug delivery system was achieved through a strategic combination of advanced materials science and innovative drug encapsulation techniques. By integrating bone morphogenetic protein-2 (BMP-2) with silver-embedded mesoporous silica nanoparticles (Ag@MSN) and encapsulating them in a silk fibroin methacryloyl (SilMA) hydrogel, the researchers created a multifunctional scaffold that not only supports bone regeneration but also provides sustained release of therapeutic agents and antibacterial properties.
The highlights of the study include the demonstration that the Ag@MSN-BMP-2/SilMA hydrogel significantly improved bone mineral density and new bone volume in a rat skull defect model, showcasing its efficacy in promoting bone regeneration. Additionally, this innovative drug delivery system exhibited effective antibacterial activity against common pathogens, addressing infection risks associated with bone defects. Overall, the findings suggest that this novel hydrogel represents a promising advancement in tissue engineering, offering a viable solution to the challenges faced in traditional bone regeneration therapies.
Reference
Mao, Yingji, et al. “A multifunctional nanocomposite hydrogel with controllable release behavior enhances bone regeneration.” Regenerative Biomaterials, vol. 10, 2023, rbad046. Oxford University Press, https://doi.org/10.1093/rb/rbad046.
