Editor: Nina
Researchers investigate the role of chemotherapy-induced extracellular heat shock protein 70 (HSP70) in enhancing tumor-associated macrophage function to promote tumor progression in breast cancer, suggesting HSP70 as a potential therapeutic target for improved patient outcomes.
Key Preview
Research Question
This study investigates how chemotherapy-induced extracellular heat shock protein 70 (HSP70) from breast cancer cells affects tumor-associated macrophages (TAMs) and contributes to tumor progression.
Research Design and Strategy
The researchers employed in vitro experiments and immunohistochemistry on 116 breast carcinoma specimens to assess the impacts of chemotherapy on HSP70 secretion and subsequent macrophage responses.
Method
The study involved coculturing breast cancer cells exposed to the chemotherapy drug epirubicin (EPI) with differentiated THP-1-derived macrophages, assessing the effects on macrophage functionality and breast cancer cell behavior.
Key Results
The findings revealed that EPI treatment of breast cancer cells increases extracellular HSP70 levels, enhancing the pro-tumorigenic effects of macrophages and promoting tumor progression through mechanisms involving transforming growth factor (TGF)-β signaling.
Significance of the Research
This research highlights the dual role of HSP70 as a marker of poor prognosis in breast cancer and suggests potential therapeutic targets to improve patient outcomes by modulating the tumor microenvironment.
Introduction
Breast cancer remains one of the most prevalent malignancies affecting women globally, with significant morbidity and mortality rates. It is characterized by the uncontrolled growth of breast cells and can present in various forms, with triple-negative breast cancer (TNBC) representing one of the most aggressive subtypes. TNBC is particularly challenging to treat due to its lack of specific hormone receptors, making it unresponsive to standard hormonal therapies. The management of breast cancer often involves a combination of surgery, radiation therapy, and systemic treatments such as chemotherapy.
A common strategy for drug delivery in traditional breast cancer treatment involves the use of chemotherapeutic agents administered systemically. These agents aim to target rapidly dividing cancer cells, thereby inhibiting tumor growth and metastasis. However, the nonspecific nature of these treatments often results in significant collateral damage to healthy tissues, leading to adverse side effects such as nausea, fatigue, and immunosuppression. Furthermore, the efficacy of chemotherapy can be compromised by the development of drug resistance, particularly in advanced stages of the disease.
The challenges associated with conventional chemotherapy are exacerbated by the tumor microenvironment (TME), which includes various cell types, such as tumor-associated macrophages (TAMs). These immune cells can promote tumor growth, angiogenesis, and metastasis, ultimately contributing to therapeutic resistance. As a result, the current treatment approaches often fall short of achieving optimal clinical outcomes, particularly for patients with aggressive breast cancer subtypes like TNBC.
To address these challenges, innovative drug delivery strategies are being explored that focus on enhancing the specificity and efficacy of treatment while minimizing systemic toxicity. One such approach involves the use of extracellular heat shock protein 70 (HSP70) as a biomarker and therapeutic target to modulate the TME. By leveraging the unique properties of HSP70, researchers aim to develop targeted therapies that can disrupt the supportive role of TAMs in tumor progression. This novel strategy represents a significant advancement in breast cancer treatment, offering the potential for improved therapeutic outcomes and enhanced patient quality of life.
Research Team and Aim
The research team for this study was led by Mio Yamaguchi-Tanaka, a prominent researcher from Tohoku University, Japan. The research was conducted over a period of time that culminated in its publication in March 2023. The findings were detailed in the paper titled “The Pro-Tumorigenic Role of Chemotherapy-Induced Extracellular HSP70 from Breast Cancer Cells via Intratumoral Macrophages,” which was published in the journal Cancers.
The aim of the research, as articulated by Yamaguchi-Tanaka, was to investigate the role of HSP70, induced by chemotherapy, in modulating macrophage behavior and its implications for breast cancer progression. Specifically, the study sought to clarify how chemotherapy-induced extracellular HSP70 affects tumor-associated macrophages (TAMs) and contributes to the pro-tumorigenic environment in breast cancer.
Experimental Process
Experiment 1: Coculture of Breast Cancer Cells and Macrophages
Primary Technique: The primary technique employed in this study was the coculture method, specifically using human breast cancer cell lines (MDA-MB-231 and MDA-MB-453) in conjunction with differentiated THP-1-derived macrophages. This approach facilitated the examination of the interactions between cancer cells and macrophages following chemotherapy treatment.
Key Steps:
- Preparation of Cell Lines: Human TNBC cell lines MDA-MB-231 and MDA-MB-453 were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).
- Macrophage Differentiation: THP-1 cells were differentiated into macrophages using phorbol 12-myristate 13-acetate (PMA) at a concentration of 20 nM for 72 hours. This differentiation was confirmed by the adherence of cells to the culture plates.
- Coculture Setup: Differentiated macrophages were then cocultured with breast cancer cells that had been treated with epirubicin (EPI) for 6 hours. The cells were washed with PBS to remove any residual drug and subsequently incubated together for 72 hours.
Data Collection and Analysis: After the coculture period, conditioned media (CM) from the macrophages were collected and analyzed for cytokine expression and macrophage activation markers. Cell proliferation and migration assays were then conducted using the CM to assess the effects of macrophage-secreted factors on breast cancer cell behavior.
Figure 1. Cell proliferation
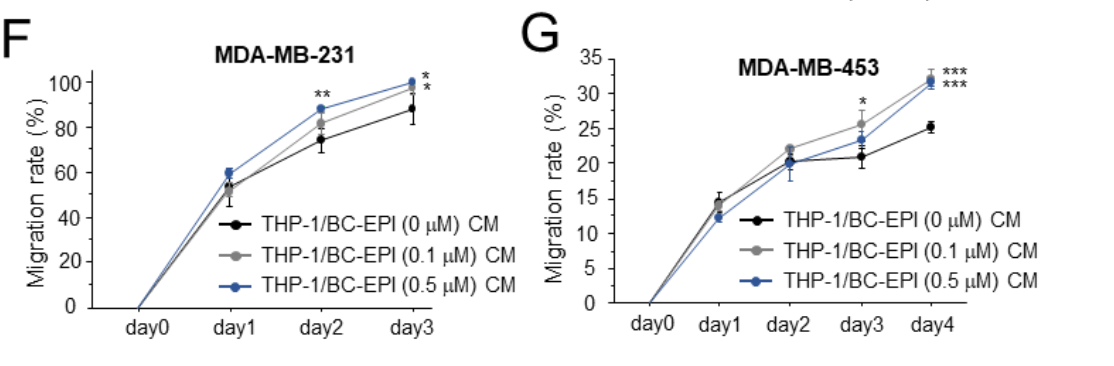
Figure 2. migration
Result: The results indicated that CM from macrophages cocultured with EPI-treated breast cancer cells significantly enhanced the proliferation, chemoresistance, and migration of breast cancer cells compared to CM from macrophages that interacted with naïve breast cancer cells.
Novel Aspect: This experiment innovatively combined the direct exposure of macrophages to EPI-treated cancer cells and subsequent analysis of their secreted factors, thus providing insights into the tumor microenvironment’s role in chemoresistance. Unlike traditional methods that isolate components, this coculture setup allowed for the observation of real-time cellular interactions and their consequences.
Experiment 2: Assessment of HSP70 Secretion
Primary Technique: Western blotting was employed to assess the levels of heat shock protein 70 (HSP70) secreted by breast cancer cells in response to chemotherapy.
Key Steps:
- Treatment of Cancer Cells: MDA-MB-231 and MDA-MB-453 cells were treated with varying concentrations of EPI (0.1 and 0.5 µM) for 24 hours.
- Collection of Conditioned Media: The CM was collected post-treatment, centrifuged at 1500 rpm for 3 minutes to remove cell debris, and stored for analysis.
- Western Blot Analysis: Protein levels of HSP70 were analyzed using SDS-PAGE followed by transfer to membranes. The membranes were probed with specific antibodies against HSP70, and the signals were visualized using enhanced chemiluminescence.
Data Collection and Analysis: The density of the HSP70 bands was quantified using ImageJ software, and levels were normalized against a loading control (β-actin).
Result: The data revealed that EPI treatment resulted in a significant increase in extracellular HSP70 levels in the CM of both breast cancer cell lines, indicating that chemotherapy promotes HSP70 secretion.
Novel Aspect: This study provided a novel link between chemotherapy and increased HSP70 secretion, utilizing Western blotting to quantify extracellular HSP70, a less commonly examined aspect in breast cancer research. This approach offers a clearer understanding of HSP70’s role in the tumor microenvironment.
Experiment 3: Functionality of Macrophages in Response to HSP70
Primary Technique: Real-time PCR was utilized to evaluate the expression of pro-tumorigenic markers in THP-1-derived macrophages following treatment with conditioned media from HSP70-silenced breast cancer cells.
Key Steps:
- Silencing HSP70: MDA-MB-231 and MDA-MB-453 cells were transfected with HSP70-specific siRNA to reduce HSP70 levels.
- Coculture and CM Collection: The CM from HSP70-silenced breast cancer cells was collected after 72 hours and used to treat THP-1-derived macrophages.
- Real-Time PCR Assay: The macrophages were harvested, and total RNA was extracted for real-time PCR analysis of CD163, TGF-β, and IL-10 expression.
Data Collection and Analysis: mRNA expression levels were normalized to RPL13A and analyzed using the ΔΔCt method to determine fold changes in expression.
Result: The results indicated that treatment with CM from HSP70-silenced breast cancer cells led to decreased expression of pro-tumorigenic markers in macrophages, particularly CD163 and TGF-β.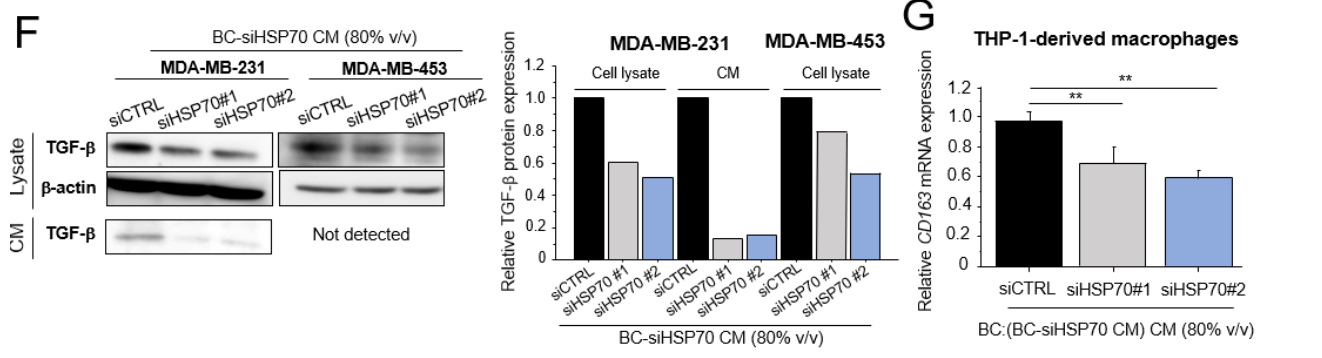
Figure 3. (F) TGF-β protein levels in cell lysate and CM from MDA-MB-231 and MDA-MB-453 cells treated with BC-siHSP70 CM (80% v/v). (G): THP-1-derived macrophages were cultured in CM from MDA-MB-231 cells treated with BC-siHSP70 CM (BC:(BC-siHSP70 CM) CM, 80% v/v), and the mRNA expression of CD163 was evaluated by real-time PCR. * p < 0.05, ** p < 0.01 vs. control (siCTRL). The data are presented as the mean ± S.D. (n = 3). BC: breast cancer, CM: conditioned medium. The uncropped blots are shown in Supplementary Materials.
Novel Aspect: This innovative approach highlights the regulatory role of HSP70 on macrophage polarization, demonstrating that silencing HSP70 directly affects macrophage functionality. This mechanism has not been extensively explored, providing potential therapeutic targets for modulating macrophage activity in the tumor microenvironment.
Experiment 4: Immunohistochemical Analysis of Clinical Samples
Primary Technique: Immunohistochemistry was the primary technique used to evaluate HSP70 expression in breast cancer tissue specimens.
Key Steps:
- Tissue Preparation: A total of 116 invasive breast carcinoma specimens were collected from patients who had undergone surgery. The tissues were fixed in formalin and embedded in paraffin.
- Staining Procedure: Sections were deparaffinized, rehydrated, and subjected to antigen retrieval. They were then incubated with HSP70 antibodies, followed by secondary antibodies conjugated with horseradish peroxidase. The reaction was visualized using a DAB substrate, and hematoxylin was used for counterstaining.
- Scoring: HSP70 immunoreactivity was evaluated based on the percentage of positive staining in the cytoplasm of breast carcinoma cells, categorized as positive if more than 10% of the cells stained positively.
Data Collection and Analysis: The immunostaining results were statistically analyzed to correlate HSP70 expression with clinicopathological parameters and patient outcomes using the Chi-square test and Kaplan-Meier survival analysis.
Result: The analysis demonstrated a significant correlation between cytoplasmic HSP70 expression and poor prognosis in breast cancer patients, particularly in relation to advanced disease stage and lymph node metastasis.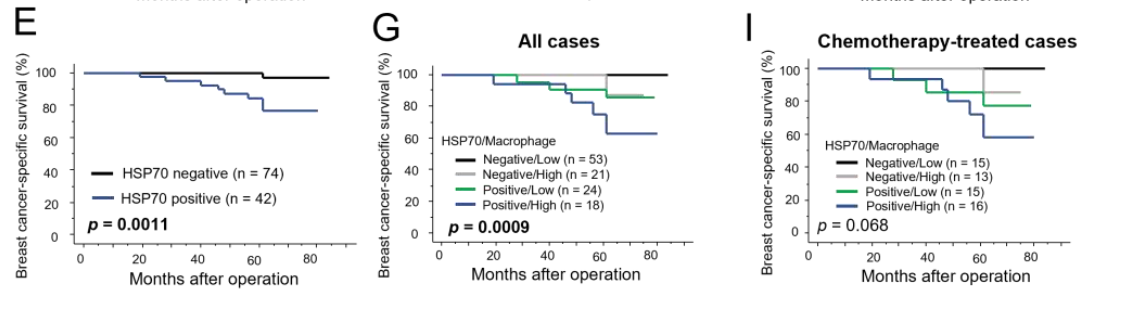
Figure 4. breast cancer -speciffc survival (E,G,I) curves according to cytoplasmic HSP70 immunoreactivity breast carcinoma cells
Novel Aspect: This experiment introduced the clinical relevance of HSP70 expression in breast cancer, providing a potential biomarker for patient prognosis. The use of immunohistochemistry in this context underscores the translational aspect of the research, bridging laboratory findings with clinical applications.
Conclusion
The successful development of an innovative drug delivery system targeting the tumor microenvironment, particularly through the modulation of extracellular heat shock protein 70 (HSP70), has been achieved by elucidating the intricate interactions between chemotherapy-induced HSP70 and tumor-associated macrophages (TAMs). By demonstrating how HSP70 influences macrophage behavior and promotes a pro-tumorigenic environment, this research provides valuable insights into potential therapeutic strategies aimed at overcoming the challenges of traditional chemotherapy.
The highlights of the study include the significant findings that chemotherapy treatment enhances the secretion of extracellular HSP70 from breast cancer cells, which in turn exacerbates the pro-tumorigenic effects of TAMs through mechanisms involving transforming growth factor (TGF)-β signaling. Additionally, the research establishes HSP70 as a marker of poor prognosis in breast cancer and underscores its potential as a therapeutic target to improve patient outcomes. By addressing the dual role of HSP70 in cancer progression, this study paves the way for the development of targeted therapies that can effectively disrupt the supportive role of TAMs, ultimately contributing to more effective breast cancer treatments.
Reference
Yamaguchi-Tanaka, Mio, et al. “The Pro-Tumorigenic Role of Chemotherapy-Induced Extracellular HSP70 from Breast Cancer Cells via Intratumoral Macrophages.” Cancers, vol. 15, no. 6, 2023, article 1903. MDPI, https://doi.org/10.3390/cancers15061903.
