Editor: Nina
Researchers develop genetically engineered cell membrane-coated nanoparticles that synergize antibacterial and immunomodulatory functions to effectively treat periodontitis and reduce inflammatory responses.
Key Preview
- Research Question: This study investigates how to effectively combine antibacterial and immunomodulatory strategies for treating periodontitis using genetically engineered cell membrane-coated nanoparticles.
- Research Design and Strategy: The researchers employed a biomimetic approach, utilizing silk fibroin nanoparticles loaded with minocycline hydrochloride, and coated with TLR4-expressing macrophage membranes to create a dual-function treatment system.
- Method: Characterization of nanoparticles was performed using techniques such as Scanning Electron Microscopy (SEM) and real-time Polymerase Chain Reaction (RT-PCR). Antibacterial and immunoregulatory effects were evaluated both in vitro and in vivo using a ligature-induced periodontitis mouse model.
- Key Results: The study found that the engineered nanoparticles effectively targeted pathogenic bacteria and significantly reduced inflammation in periodontal tissues, demonstrating both antibacterial and immunoregulatory effects.
- Significance of the Research: This research introduces a novel therapeutic strategy that integrates precise drug delivery with immune modulation, potentially improving outcomes for patients suffering from periodontitis and other bacterial infections.
Introduction
Periodontitis is a prevalent inflammatory disease primarily caused by bacterial infection of the supporting structures of the teeth, leading to the destruction of periodontal tissues and, ultimately, tooth loss. Beyond its local effects, periodontitis is associated with systemic health issues, including cardiovascular disease, diabetes, and respiratory conditions. The pathogenesis of periodontitis is characterized by a complex interplay between pathogenic bacteria, host immune responses, and inflammatory processes, making effective management of the disease particularly challenging.
Traditional treatment strategies for periodontitis often involve mechanical debridement and the use of systemic or local antimicrobial agents. These approaches aim to reduce bacterial load and manage inflammation through the delivery of antibiotics or antiseptics to the affected periodontal sites. However, conventional drug delivery methods face several significant challenges, including incomplete drug penetration into deep periodontal pockets and the rapid re-colonization of pathogens following treatment. Additionally, the systemic use of antibiotics can lead to side effects, such as gastrointestinal disturbances and the development of antibiotic resistance, which further complicates the management of periodontitis.
The limitations of traditional therapies highlight the need for more effective and targeted treatment strategies that can address both the bacterial infection and the immune dysregulation associated with periodontitis. In this context, the innovative use of engineered drug delivery systems, such as genetically engineered cell membrane-coated nanoparticles, presents a promising alternative. This novel approach combines the advantages of targeted drug delivery with immune modulation, allowing for precise localization of therapeutic agents to inflamed tissues while simultaneously enhancing the immune response against pathogenic bacteria. By leveraging the innate recognition properties of toll-like receptors (TLR4), these advanced delivery systems aim to improve treatment outcomes for patients suffering from periodontitis and other related inflammatory diseases.
Research Team and Aim
The research team was led by Dr. Sheng Yang, a distinguished researcher at Chongqing Medical University, where the study was conducted in 2022. The team comprised co-authors Yangjia Deng, Mingxing Ren, Ping He, Fengyi Liu, Xu Wang, Chongjing Zhou, and Yuzhou Li. Their collaborative work culminated in the publication of the paper titled “Genetically engineered cell membrane-coated nanoparticles for antibacterial and immunoregulatory dual-function treatment of ligature-induced periodontitis” in the journal Frontiers in Bioengineering and Biotechnology.
The aim of this research, as stated by Dr. Yang, was to “overcome the problem that conventional pharmacological treatments of periodontitis cannot effectively synergize antimicrobial and immunomodulation.” This study aimed to introduce a novel therapeutic approach that combines targeted antibacterial action with immune modulation to enhance the management of periodontitis.
Experimental Process
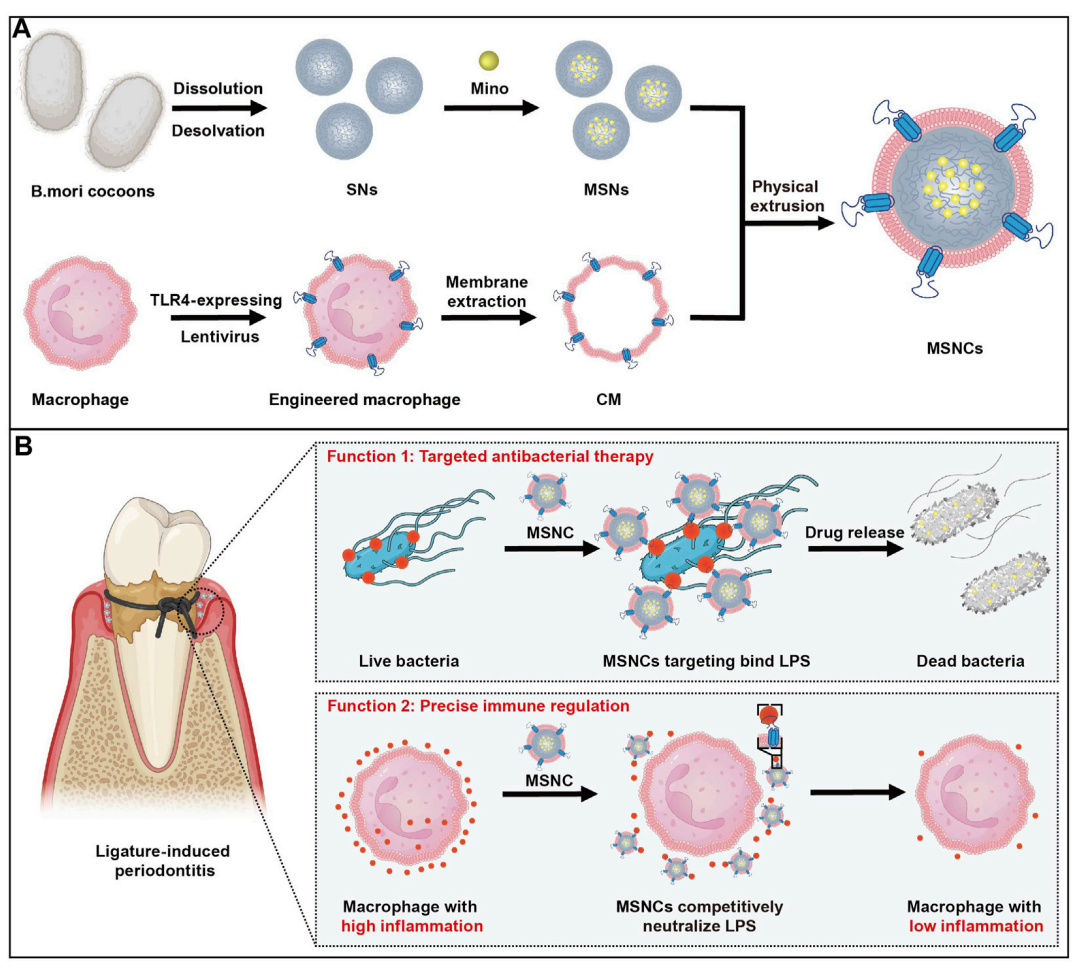
Figure 1. The fabrication and application of functioned cell membrane coated silk fibroin nanoparticles loaded with minocycline hydrochloride
Experiment 1: Preparation of Silk Fibroin Nanoparticles (SNs)
Primary Technique: The primary technique employed in this experiment was the desolvation method for synthesizing silk fibroin nanoparticles.
Key Steps:
- B.mori cocoons were cut into small pieces and boiled in 0.02 M sodium carbonate for 60 minutes to remove sericin.
- The degummed silk fibroin was dissolved in 9.3 M lithium bromide at 60°C for 4 hours.
- The solution was dialyzed in double-distilled water for 72 hours to remove lithium bromide.
- The silk fibroin solution was centrifuged at 5,000 rpm to remove any undissolved materials.
- A 5% silk fibroin solution was added dropwise to acetone, leading to the precipitation of nanoparticles.
- The nanoparticles were collected by centrifugation at 32,000 g for 2 hours and then resuspended in double-distilled water.
Data Collection and Analysis: The morphology of the SNs was analyzed using scanning electron microscopy (SEM). The particle size and zeta potential were measured via dynamic light scattering (DLS). Additionally, cytotoxicity was assessed using a cell counting kit-8 (CCK-8).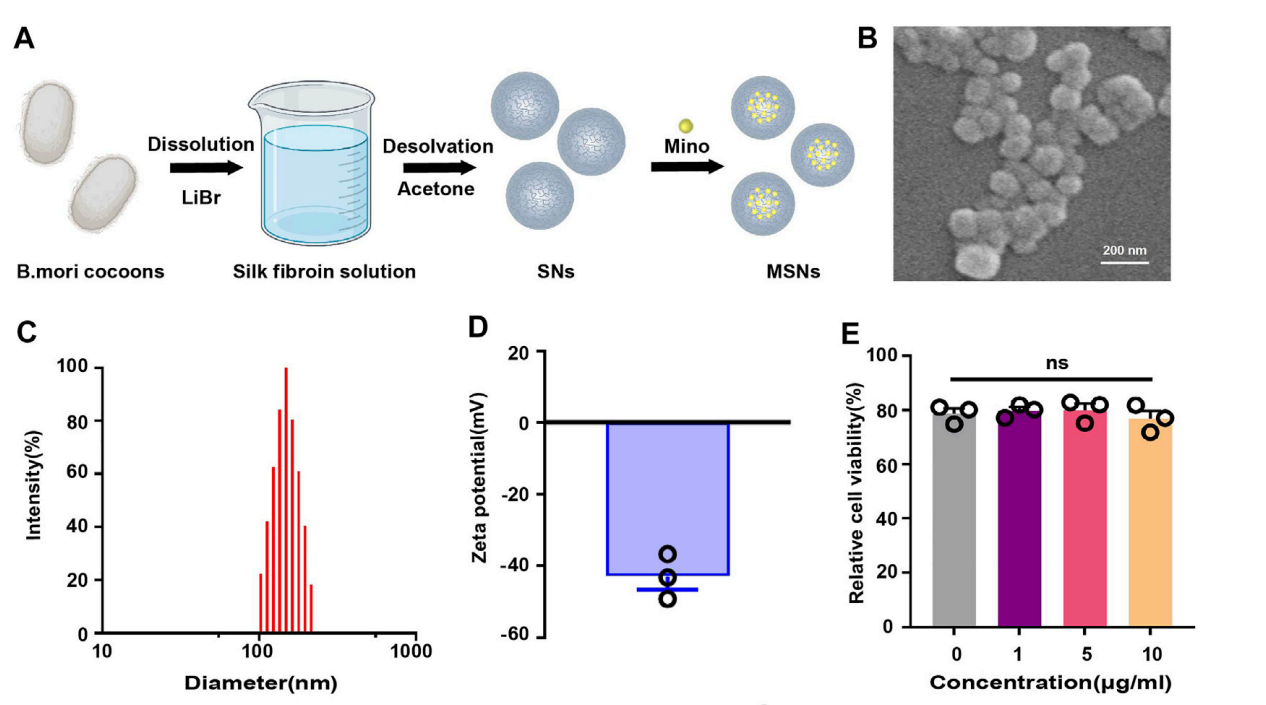
Figure 2. (B) Scanning electron microscopy of SNs. © Particle size distribution of SNs. (D) Zeta potential diagram of SNs. (E) CCK-8 of SNs.
Result: The SEM images confirmed the spherical morphology of the nanoparticles, with an average diameter of approximately 150 nm. The DLS results indicated a size of 114.63 ± 4.55 nm and a zeta potential of -39.70 ± 2.77 mV, demonstrating favorable characteristics for drug delivery.
Novel Aspect: This experiment established a biocompatible and biodegradable nanoparticle system using silk fibroin, which is advantageous over traditional synthetic polymers due to its natural origin and reduced cytotoxicity.
Experiment 2: Loading of Minocycline Hydrochloride into SNs
Primary Technique: The primary technique used in this experiment was drug loading through physical mixing.
Key Steps:
- Different mass ratios of minocycline hydrochloride (Mino) to SNs (0.125, 0.375, 0.5) were prepared by adding the corresponding mass of Mino to the SNs suspension.
- The mixture was stirred overnight at room temperature, protected from light.
- The supernatant was collected after centrifugation at 32,000 g for 30 minutes to separate unencapsulated Mino.
- The drug loading efficiency and encapsulation efficiency were calculated based on the initial amount of Mino and the amount remaining in the supernatant.
Data Collection and Analysis: The encapsulation and loading efficiencies were calculated using the following formulas:
- Drug loading efficiency = (Weight of fed drug – Drug amount in supernatant) / Weight of dry NPs × 100%
- Encapsulation efficiency = (Weight of fed drug – Drug amount in supernatant) / Weight of fed drug × 100%
Result: The optimal mass ratio of Mino to SNs was determined to be 0.375, yielding a drug loading efficiency of 7.86% and an encapsulation efficiency of 20.36%.
Novel Aspect: This method of drug loading into silk fibroin nanoparticles demonstrated improved loading capacities compared to conventional methods, enhancing the potential for effective localized delivery.
Experiment 3: Genetic Engineering of TLR4-Expressing RAW264.7 Macrophages
Primary Technique: The primary technique utilized was lentiviral transfection for genetic modification.
Key Steps:
- RAW264.7 cells were cultured and incubated with TLR4 lentivirus (MOI = 50) for 10 hours.
- After 48 hours, the cells were harvested for analysis of TLR4 expression using real-time polymerase chain reaction (RT-PCR) and Western blotting.
- Cells were selected using puromycin (4 μg/mL) for 2 days to ensure stable expression of TLR4.
Data Collection and Analysis: TLR4 mRNA levels were quantified using RT-PCR, and protein expression was confirmed through Western blot analysis.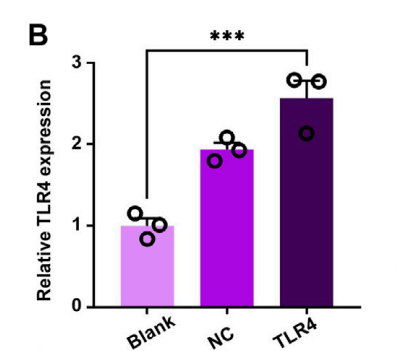
Figure 3. (B) TLR4-expressing in RAW264.7 cells by RT-PCR.
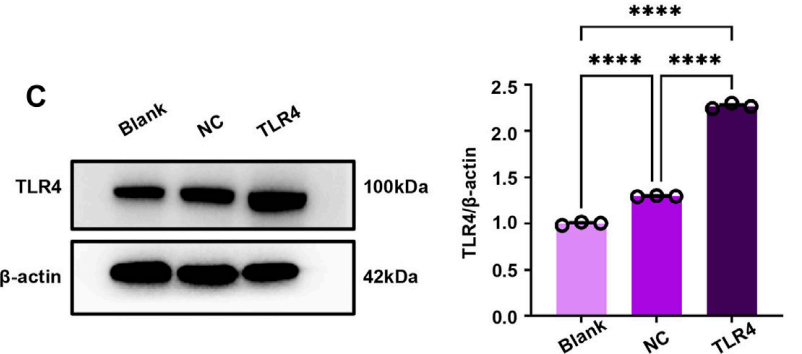
Figure 4. © TLR4-expressing in RAW264.7 cells by WB. (D) Quantitative analysis of the WB.
Result: The TLR4-expressing RAW264.7 cells showed a significant increase in TLR4 mRNA and protein levels compared to control groups, confirming successful genetic modification.
Novel Aspect: This experiment established TLR4-expressing macrophages as an innovative approach for enhancing immune recognition and response, which is a significant improvement over traditional macrophage models lacking specific receptor expression.
Experiment 4: Coating of SNs with TLR4-Expressing Macrophage Membranes
Primary Technique: The primary technique employed was membrane extrusion to coat nanoparticles.
Key Steps:
- Cell membranes were extracted from TLR4-expressing RAW264.7 macrophages using a membrane protein extraction kit.
- The extracted membranes were mixed with SNs at a 1:1 mass ratio.
- The mixture was extruded through a polycarbonate membrane using a liposome extruder to create MSNCs (macrophage membrane-coated silk fibroin nanoparticles).
Data Collection and Analysis: The successful coating was confirmed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The size and zeta potential of MSNCs were also measured via DLS.
Result: The MSNCs exhibited a distinct ring-like structure, indicative of successful membrane coating, with a particle size of 134.04 ± 1.50 nm and a zeta potential of -36.09 ± 0.98 mV.
Novel Aspect: This study introduced a novel bionic approach by utilizing TLR4-expressing macrophage membranes for nanoparticle coating, greatly enhancing targeting capabilities and immune modulation compared to conventional nanoparticle delivery systems.
Experiment 5: Evaluation of Antibacterial and Immunoregulatory Effects of MSNCs
Primary Technique: The primary technique used was in vitro and in vivo testing to assess the effects of MSNCs.
Key Steps:
- In vitro studies were conducted by incubating E. coli with MSNCs and assessing bacterial viability through agar plate assays.
- For immunoregulatory effects, RAW264.7 cells were treated with LPS in the presence of MSNCs, and gene expression of pro-inflammatory markers was measured by RT-PCR.
- In vivo studies involved administering MSNCs to a ligature-induced periodontitis mouse model, followed by collection of periodontal tissues for analysis.
Data Collection and Analysis: Bacterial viability was quantified by counting colonies on agar plates. RT-PCR was used to measure expression levels of IL-1β and IL-6, and statistical analysis was performed using one-way ANOVA.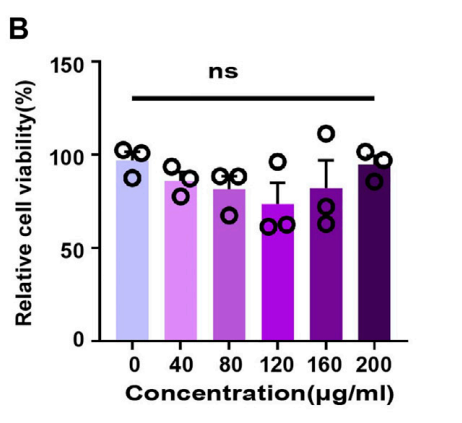
Figure 5. CCK-8 of SNCs.
Result: MSNCs demonstrated significant antibacterial effects, reducing E. coli viability, and effectively inhibited the expression of pro-inflammatory cytokines in macrophages. In vivo, MSNCs significantly reduced periodontal inflammation and bone loss in the mouse model.
Novel Aspect: The combination of antibacterial and immunoregulatory functions in a single nanoparticle system offers a multifaceted therapeutic strategy for periodontitis, representing a significant advancement over traditional therapies that target only one aspect of the disease.
Conclusion
This study successfully demonstrates the development of a genetically engineered cell membrane-coated nanoparticle system (MSNCs) that effectively targets pathogenic bacteria while modulating the immune response in the treatment of periodontitis. The successful creation of this drug delivery system was achieved through a biomimetic approach, utilizing silk fibroin nanoparticles loaded with the antibacterial agent minocycline hydrochloride and coated with TLR4-expressing macrophage membranes. This innovative design not only enhances the targeting capabilities of the nanoparticles but also allows for the simultaneous neutralization of lipopolysaccharide (LPS), thereby reducing inflammatory responses.
A key highlight of the study is the dual functionality of MSNCs, which combines precise antibacterial action with immunoregulatory effects. The findings indicate that these engineered nanoparticles significantly reduce inflammation in periodontal tissues and effectively inhibit bacterial growth, offering a multifaceted therapeutic strategy for managing periodontitis. This novel approach has the potential to improve treatment outcomes for patients suffering from periodontitis and other related bacterial infections, paving the way for future advancements in targeted drug delivery systems.
Reference
Deng, Yangjia, et al. “Genetically Engineered Cell Membrane-Coated Nanoparticles for Antibacterial and Immunoregulatory Dual-Function Treatment of Ligature-Induced Periodontitis.” Frontiers in Bioengineering and Biotechnology, vol. 11, 2023, article no. 1113367, doi:10.3389/fbioe.2023.1113367.
