Editor: Nina
Researchers develop a tacrolimus and thymoquinone co-loaded nanostructured lipid carrier gel to enhance the topical treatment of psoriasis through improved drug delivery and sustained release.
Key Preview
- Research Question
The study investigates the development of a novel gel formulation using tacrolimus and thymoquinone co-loaded into nanostructured lipid carriers (NLCs) for enhanced topical treatment of psoriasis. - Research Design and Strategy
The research employs a Box-Behnken design to optimize various formulation parameters, including lipid and surfactant concentration, to achieve the desired physicochemical properties of the gel. - Method
The formulation was prepared using the emulsification-solvent evaporation technique, followed by characterization through various analytical methods, including particle size determination, entrapment efficiency, in vitro release studies, and cytotoxicity assays. - Key Results
The optimized TAC-THQ-NLCs showed a particle size of 144.95 nm, a polydispersity index of 0.160, and an entrapment efficiency greater than 70% for both drugs. The gel formulation demonstrated sustained drug release and enhanced skin permeation compared to conventional gels. - Significance of the Research
This study presents a promising combinatorial approach for psoriasis treatment, potentially improving drug delivery and reducing side effects through targeted therapy.
Introduction
Psoriasis is a chronic autoimmune skin disorder characterized by the rapid proliferation of keratinocytes, leading to the development of thick, scaly patches on the skin. It affects approximately 2-4% of the global population and is often accompanied by significant physical discomfort and psychological distress. The pathophysiology of psoriasis involves a complex interplay of genetic, environmental, and immunological factors, resulting in inflammation and dysregulation of the skin barrier. Traditional treatments for psoriasis primarily include topical corticosteroids, vitamin D analogues, and systemic therapies. These treatments aim to reduce inflammation and normalize keratinocyte proliferation; however, they often face limitations in efficacy and patient adherence.
A common strategy in traditional treatment involves the topical application of medications directly to affected areas. While this approach allows for localized drug delivery with minimal systemic exposure, it is frequently hindered by the inherent challenges posed by the psoriatic skin. The thickened and scaly nature of psoriatic plaques acts as a significant barrier to drug penetration, resulting in suboptimal therapeutic outcomes. Moreover, the variable absorption rates of topical formulations can lead to inconsistent efficacy, further complicating disease management.
These challenges ultimately result in inadequate therapeutic responses, increased treatment costs, and a lower quality of life for patients suffering from psoriasis. The limitations of conventional drug delivery systems necessitate the exploration of more advanced strategies that can enhance drug permeation and provide sustained therapeutic effects.
In light of these challenges, innovative drug delivery strategies employing nanotechnology have emerged as promising solutions. One such strategy involves the use of nanostructured lipid carriers (NLCs), which can encapsulate therapeutic agents and facilitate targeted delivery to the skin. By improving the solubility and bioavailability of lipophilic drugs, NLCs offer enhanced drug penetration through the stratum corneum, allowing for more effective treatment of psoriasis. This study explores the formulation of a novel gel comprising tacrolimus (TAC) and thymoquinone (THQ) co-loaded in NLCs, aiming to leverage their synergistic effects for improved therapeutic outcomes in psoriasis management.
Research Team and Aim
The research team for this study comprises Meraj Alam, Md. Rizwanullah, Showkat R. Mir, and Saima Amin, all affiliated with the Department of Pharmaceutics at the School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi, India. The lead researcher, Meraj Alam, conducted this research, which was published in June 2023. The study is titled “Statistically Optimized Tacrolimus and Thymoquinone Co-Loaded Nanostructured Lipid Carriers Gel for Improved Topical Treatment of Psoriasis” and was published in the journal Gels.
The primary aim of the research, as articulated by the lead researcher, is to “formulate and optimize a co-loaded gel of TAC and THQ using NLCs to improve the topical treatment of psoriasis.” This objective underscores the team’s commitment to developing an innovative drug delivery system that enhances therapeutic efficacy and patient adherence for individuals suffering from this chronic skin condition.
Experimental Process
Primary Technique: Emulsification-Solvent Evaporation
The primary technique utilized in this research was the emulsification-solvent evaporation method, which is pivotal for the formulation of nanostructured lipid carriers (NLCs). This method allows for the effective encapsulation of lipophilic drugs, improving their solubility and bioavailability, especially for topical applications.
Experiment 1: Formulation of TAC-THQ-NLCs
Key Steps:
- Preparation of Organic Phase: Dissolve solid lipid (glyceryl monostearate) and liquid lipid (Capryol 90) in dichloromethane (DCM) while heating to 60 °C. Add tacrolimus (TAC) and thymoquinone (THQ) to this mixture to ensure complete solubilization.
- Preparation of Aqueous Phase: In a separate vessel, prepare the aqueous phase by dissolving a mixture of surfactants (Tween 80 and Span 20) in distilled water, maintaining a temperature of 25 °C.
- Emulsification: Gradually add the organic phase to the aqueous phase under continuous stirring at 1000 rpm to form a water-in-oil (W/O) emulsion.
- Solvent Evaporation: Stir the emulsion for 8 hours at room temperature to allow DCM to evaporate, resulting in the formation of TAC-THQ-NLCs.
Data Collection and Analysis:
- The particle size (PS) and polydispersity index (PDI) of the NLCs were measured using dynamic light scattering (DLS). Each sample was diluted 20 times with deionized water for analysis.
- Statistical optimization was performed using Design-Expert software to evaluate the formulation parameters.
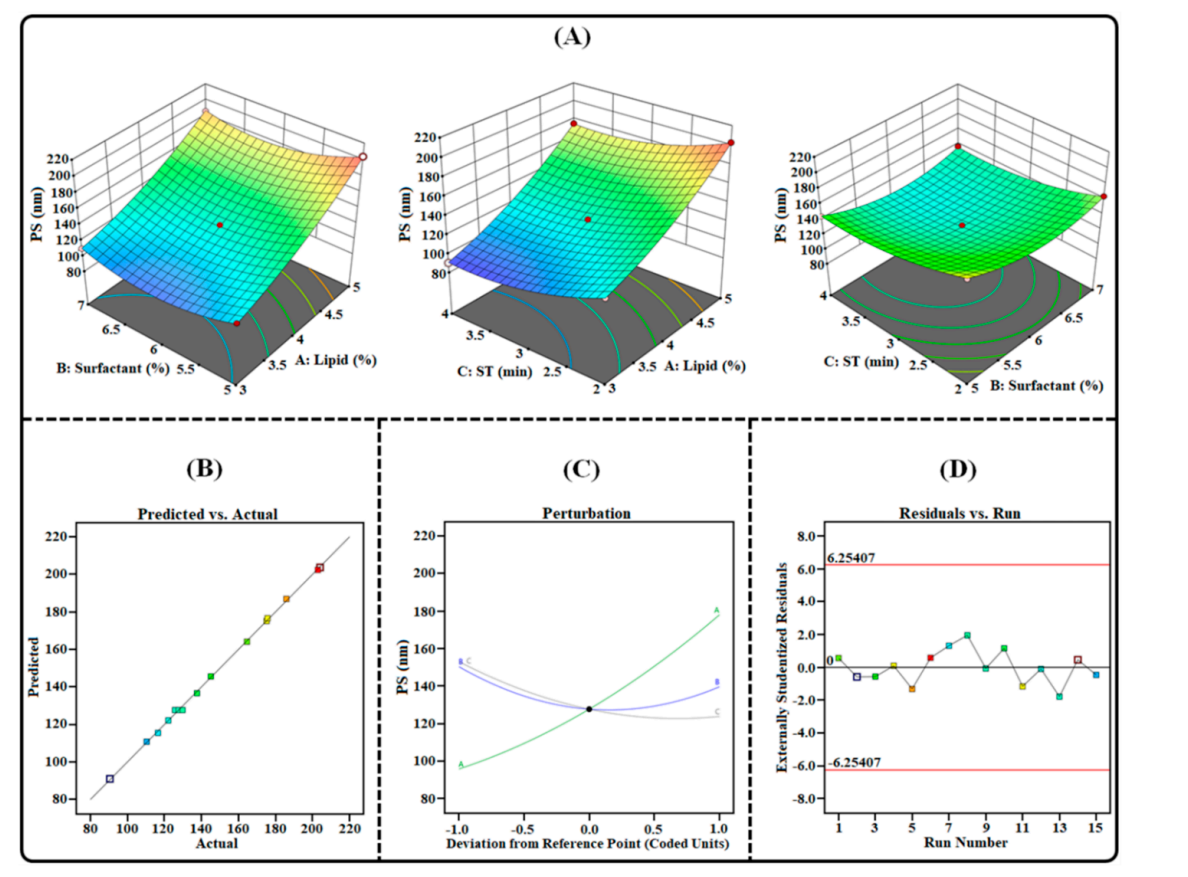
Figure 1. Image illustrating the impact of independent factors on PS: (A) 3D surface plot; (B) predicted vs. actual response; © perturbation plot; and (D) residual vs. run plot of TAC-THQ-NLCs optimized BBD.
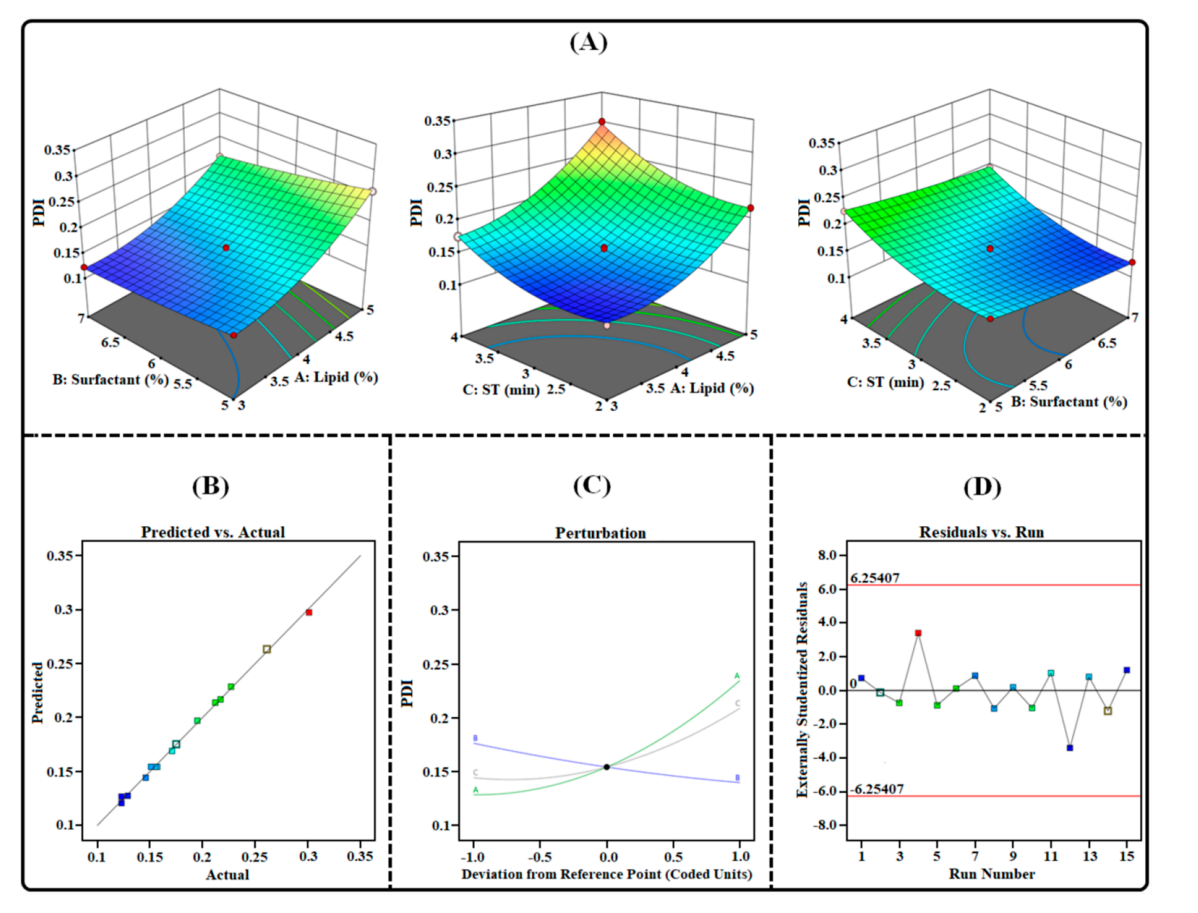
Figure 2. Image illustrating the impact of independent factors on PDI: (A) 3D Surface plot; (B) predicted vs. actual response; © perturbation plot; and (D) residual vs. run plot of TAC-THQ-NLCs optimized by BBD.
Result:
- The optimized formulation yielded a particle size of 144.95 ± 2.80 nm and a PDI of 0.160 ± 0.021, suggesting a narrow particle size distribution conducive for skin penetration.
Novel Aspect:
- The formulation of TAC and THQ in a single NLC system represents a significant advancement over traditional single-drug approaches, potentially enhancing therapeutic efficacy and minimizing adverse effects associated with monotherapy.
Experiment 2: Characterization of TAC-THQ-NLCs
Key Steps:
- Zeta Potential Measurement: Analyze the zeta potential of the optimized NLCs using a zeta sizer. Dilute the NLCs as previously described and place them in an electrophoretic cell for assessment.
- Entrapment Efficiency Calculation: Employ ultracentrifugation at 15,000 rpm for 15 minutes to separate free drugs from encapsulated drugs. Analyze the supernatant by RP-HPLC to quantify unencapsulated drug concentrations.
Data Collection and Analysis:
- The zeta potential was recorded to assess the stability of the NLCs. The entrapment efficiency (%EE) was calculated using the formula:
[ %EE = \left( \frac{\text{Total drug} – \text{Unencapsulated drug}}{\text{Total drug}} \right) \times 100 ] - Data were subjected to statistical analysis to validate the robustness of the results.
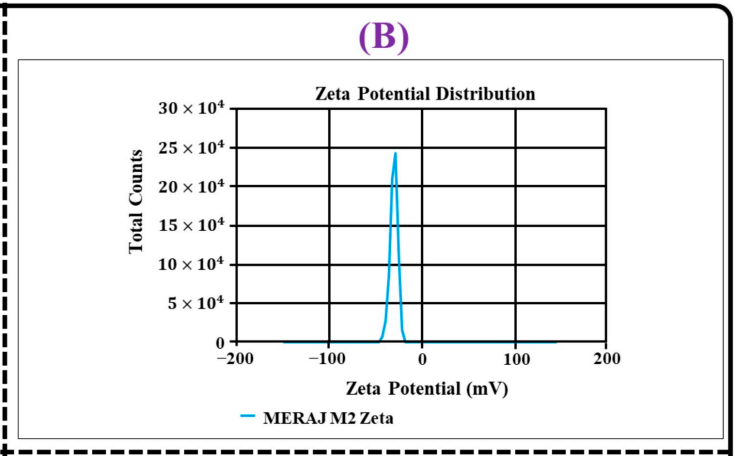
Figure 3. (B) zeta potential distribution
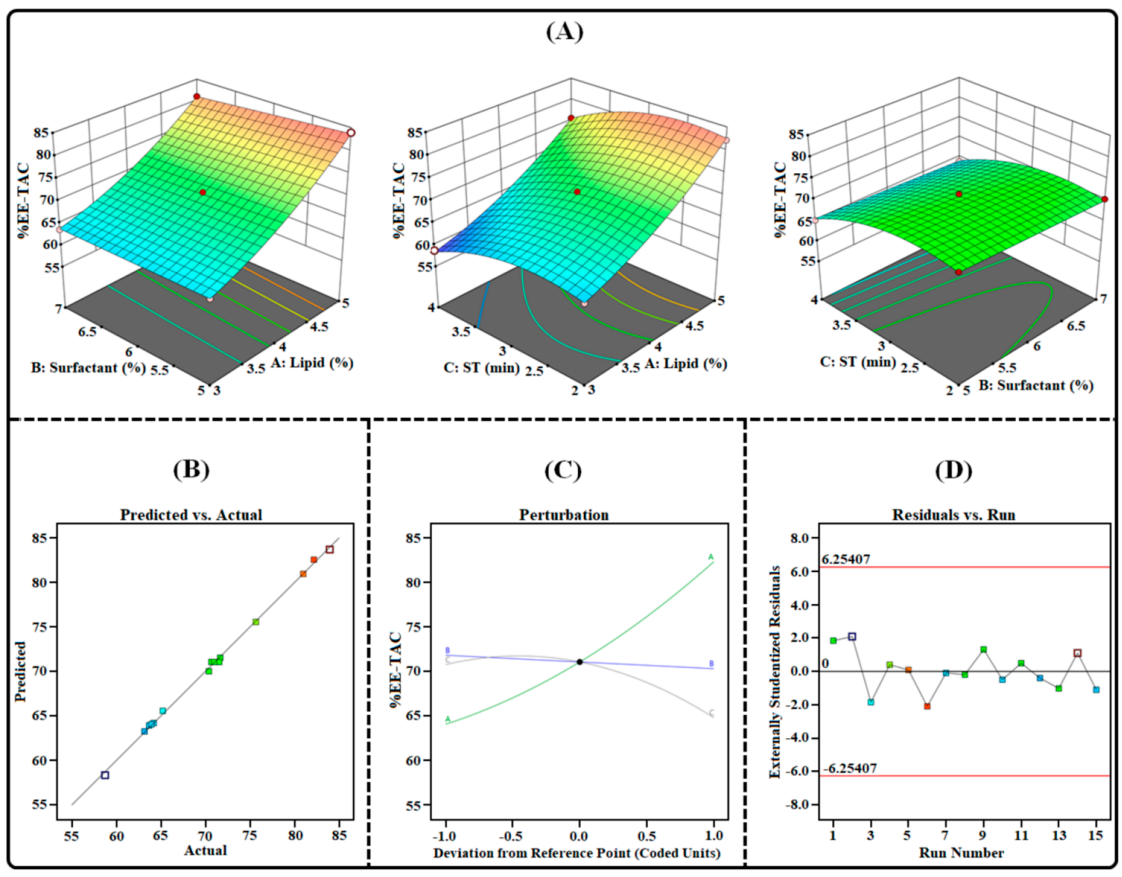
Figure 4. Image illustrating the impact of independent factors on the %EE-TAC: (A) 3D surface plot; (B) predicted vs. actual response; © bation plot; and (D) residual vs. run plot of TAC-THCs optimized by BBD.
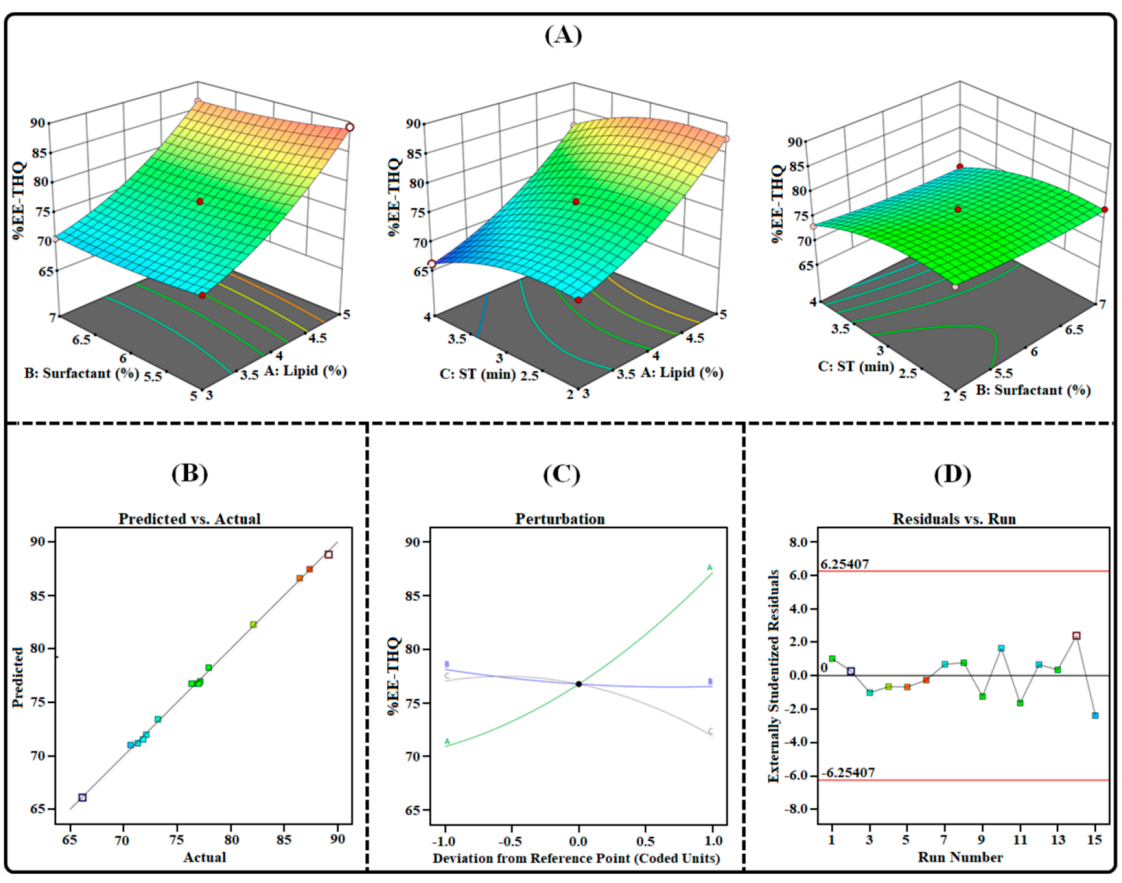
Figure 5. mage illustrating the impact of independent factors on the %EE-THQ: (A) three-dimensional surface plot; (B) predicted vs. actual response; © perturbation plot; and (D) residual vs. run plot of TAC-THQ-NLCs optimized by BDD.
Result:
- The %EE for TAC and THQ was found to be 73.44 ± 2.54% and 78.62 ± 2.75%, respectively, with a zeta potential of -29.47 ± 1.9 mV, indicating a stable and well-formed nanocarrier system.
Novel Aspect:
- The high entrapment efficiency and favorable zeta potential highlight the potential of these NLCs for effective drug delivery, outperforming conventional formulations that often struggle with stability and drug leakage.
Experiment 3: In Vitro Drug Release Studies
Key Steps:
- Preparation of Release Setup: Place the TAC-THQ-NLCs in a dialysis bag and immerse it in a release medium (PBS with 0.5% Tween 80) at 37 ± 1 °C.
- Sampling: Withdraw samples at predetermined intervals (e.g., 1, 2, 4, 8, 12, and 24 hours) and replace with fresh medium to maintain sink conditions.
Data Collection and Analysis:
- Measure the cumulative percentage of drug released at each time point using RP-HPLC. The data were analyzed to fit into various kinetic models (zero-order, first-order, Higuchi, and Korsmeyer-Peppas) to elucidate the release mechanism.
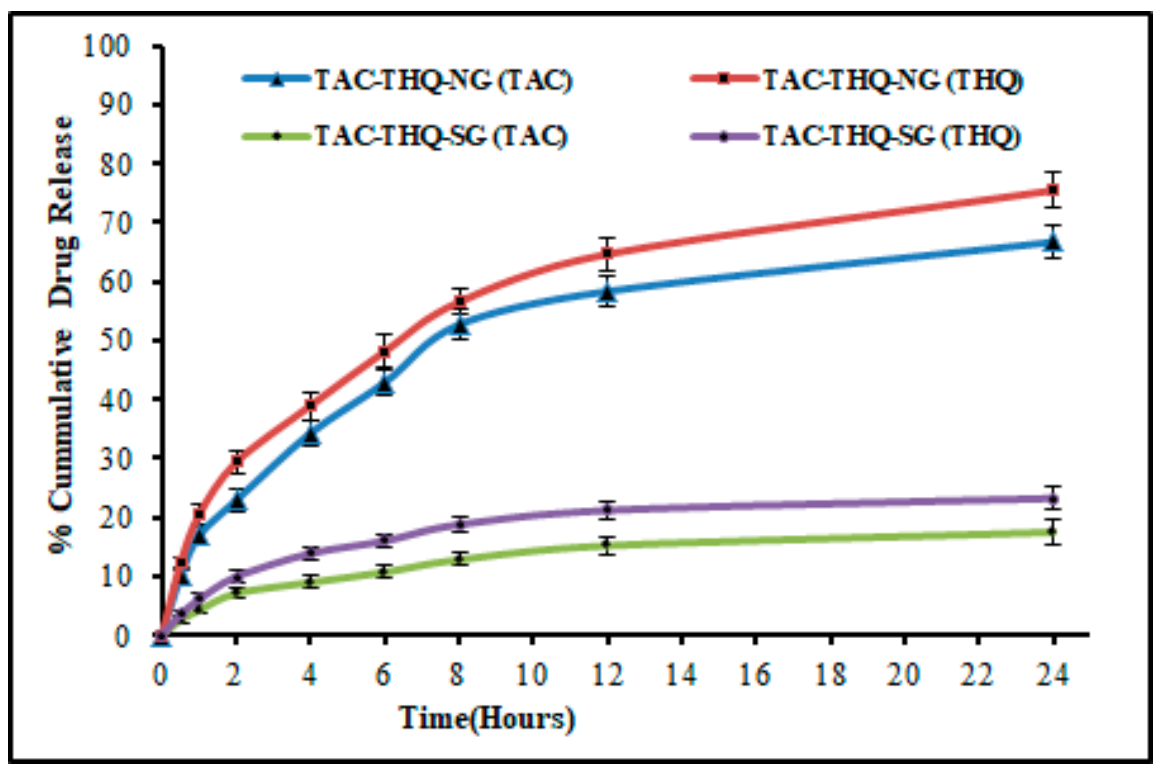
Figure 6. Comparative in vitro drug-release profiles of TAC and THQ from the optimized TACTHQ-NG and TAC-THQ-SG.
Result:
- The TAC-THQ-NLCs exhibited a sustained release profile over 24 hours, aligning with the Korsmeyer-Peppas model, indicative of a Fickian diffusion mechanism.
Novel Aspect:
- The sustained release achieved in this formulation is a substantial improvement over traditional topical formulations, which typically exhibit burst release profiles, limiting their therapeutic effectiveness.
Experiment 4: Cytotoxicity Assessment via MTT Assay
Key Steps:
- Cell Culture: Cultivate HaCaT cells (human keratinocyte cell line) in DMEM medium supplemented with 10% fetal bovine serum (FBS) at 37 °C in a CO2 incubator.
- Treatment: Expose the cells to varying concentrations of TAC-THQ-NG and TAC-THQ-SG formulations for 24 hours.
- MTT Assay: After treatment, add MTT solution to each well and incubate for 2 hours to allow for formazan crystal formation.
Data Collection and Analysis:
- Measure the absorbance at 570 nm to determine cell viability. Calculate IC50 values using dose-response curves to assess the antiproliferative effects of each formulation.
Result:
- The IC50 values were 0.1303 µg/mL for TAC-THQ-NG, significantly lower than 18.93 µg/mL for TAC-SG, indicating enhanced cytotoxicity of the combination formulation against hyperproliferative keratinocytes.
Novel Aspect:
- The marked increase in cytotoxicity of the dual-drug formulation compared to single-drug formulations underscores the therapeutic potential of TAC-THQ-NLCs in effectively targeting psoriasis.
Conclusion
The successful development of the tacrolimus and thymoquinone co-loaded nanostructured lipid carriers (TAC-THQ-NLCs) for the topical treatment of psoriasis was achieved through a systematic and innovative approach that integrated advanced nanotechnology with rigorous experimental design. By employing the emulsification-solvent evaporation technique and optimizing various formulation parameters using a Box-Behnken design, the research team was able to create an effective delivery system that significantly enhances drug solubility and stability.
Key highlights of the study include the formulation’s ability to demonstrate a sustained drug release profile, with TAC and THQ showing high entrapment efficiency of 73.44% and 78.62%, respectively. Additionally, the TAC-THQ-NLCs exhibited a particle size of 144.95 nm and a polydispersity index of 0.160, which are conducive for skin penetration and enhanced therapeutic efficacy. The in vitro cytotoxicity assessment revealed that the combination formulation was significantly more effective in inhibiting the proliferation of hyperproliferative keratinocytes compared to single-drug formulations, underscoring the therapeutic potential of TAC-THQ-NLCs in improving the management of psoriasis.
Overall, this study presents a promising combinatorial approach for psoriasis treatment, paving the way for future research to validate the in vivo efficacy of this novel drug delivery system and explore broader applications in dermatological therapies.
Reference
Alam, Meraj, et al. “Statistically Optimized Tacrolimus and Thymoquinone Co-Loaded Nanostructured Lipid Carriers Gel for Improved Topical Treatment of Psoriasis.” Gels, vol. 9, no. 7, 2023, p. 515. MDPI, https://doi.org/10.3390/gels9070515.
