Editor: Tiffany
A study reveals a novel nanocomposite that significantly improves the delivery of chemotherapy drugs and gene therapy agents to cancer cells, potentially revolutionizing cancer treatment methodologies.
Key Highlights:
- Research Question:
Can a newly engineered nanocomposite enhance the delivery of both doxorubicin and CRISPR to cancer cells? - Research Difficulties:
Challenges include ensuring effective cellular uptake of the nanocomposite and achieving a high transfection efficiency in a physiologically relevant environment. - Key Findings:
The engineered nanocomposite successfully co-delivers doxorubicin and pCRISPR, with a transfection efficiency of up to 38.3% in A549 lung cancer cells and a hemolysis rate of less than 1%, indicating high biosafety. - Innovative Aspects:
Utilization of amine-functionalized metal-organic frameworks combined with a cell-imprinted substrate to mimic the cellular environment significantly enhances drug and gene delivery efficacy. - Importance of the Study:
This research contributes to the advancement of combination therapies in cancer treatment, offering a promising platform for targeted and efficient delivery of therapeutic agents.
Contextualizing Cancer Therapy and Delivery Challenges
Cancer is a complex and multifaceted disease characterized by the uncontrolled growth of abnormal cells. It can arise in virtually any tissue of the body and is often classified into different types based on the origin of the cancerous cells, such as carcinomas, sarcomas, leukemias, and lymphomas. The symptoms of cancer vary greatly depending on the type and stage of the disease, but common manifestations include unexplained weight loss, persistent fatigue, pain, and changes in skin appearance or function. Current therapeutic options, such as surgery, chemotherapy, and radiation, often have significant limitations, including severe side effects, non-specific targeting of healthy cells, and the potential for drug resistance. As the field of cancer treatment evolves, there is a pressing need for innovative approaches that can improve the efficacy and safety of therapies. Combination therapies that integrate both drug delivery and gene therapy represent a promising strategy to enhance treatment outcomes by targeting the cancer cells more precisely while minimizing harm to surrounding healthy tissues. This study addresses these challenges by exploring a novel nanocomposite designed to deliver chemotherapy and gene-editing tools concurrently, potentially revolutionizing cancer treatment paradigms.
Objectives of Nanocomposite Development for Cancer Treatment
The primary aim of this research is to develop an innovative nanocomposite that effectively co-delivers the chemotherapy drug doxorubicin (DOX) and the gene-editing agent pCRISPR to cancer cells, thereby enhancing therapeutic efficacy while minimizing side effects. Specifically, the study seeks to investigate the properties and performance of the engineered nanocomposite, which combines amine-functionalized metal-organic frameworks (MIL-125(Ti)) with a copolymer and manganese ferrite nanoparticles. The main
objectives of the research include:
- Characterization of the Nanocomposite: To assess the structural and functional characteristics of the engineered nanocomposite, including its drug-loading capacity, surface properties, and biosafety profile.
- Evaluation of Cellular Uptake and Transfection Efficiency: To determine how surface modification with amine groups enhances cellular uptake of the nanocomposite and improves transfection efficiency of pCRISPR in A549 lung cancer cells.
- Assessment of Therapeutic Efficacy: To evaluate the combined effects of DOX and pCRISPR on cancer cell viability and gene expression, establishing the potential for synergistic treatment outcomes.
- Investigation of Hemocompatibility: To analyze the hemolysis rate of the nanocomposite, ensuring its safety for potential in vivo applications.
- Exploration of the Cell-Imprinted Substrate: To assess the role of an engineered cell-imprinted substrate in facilitating drug and gene delivery in a physiologically relevant microenvironment, aiming for improved therapeutic efficacy.
Methodological Framework and Key Experimental Outcomes
1. Experimental Process Outline:
- Design and fabrication of the nanocomposite.
- Characterization of nanocomposite properties (FT-IR, XRD, FESEM, DLS, zeta potential).
- Synthesis of manganese ferrite nanoparticles.
- Synthesis of MIL-125(Ti) and NH2-MIL-125(Ti).
- Loading of doxorubicin (DOX) into the nanocomposite.
- Decoration of pCRISPR onto the surface of the nanocomposite.
- In vitro biocompatibility assessment using MTT assay.
- Development of the cell-imprinted substrate for enhanced delivery.
- Evaluation of gene expression through transfection efficacy studies in A549 lung cancer cells.
- Hemolysis testing to assess biosafety.
2. Key Experiments:
Experiment 1: Synthesis of Nanocomposite
- Procedure: The nanocomposite was synthesized through an in situ copolymerization method, combining amine-functionalized MIL-125(Ti), poly(aniline-co-para-phenylenediamine), and manganese ferrite nanoparticles. The components were mixed under controlled conditions with ammonium persulfate as the initiator.
- Result: The final products were characterized using various techniques, revealing successful synthesis without significant degradation of the components.
- Finding: The synthesized nanocomposite displayed a well-defined structure and high stability, setting the stage for effective drug and gene delivery applications.
Experiment 2: Drug Loading Efficiency
- Procedure: The drug loading capacity of the nanocomposite was assessed by incubating the nanocarriers with DOX at different weight ratios (1:1, 1:2, and 2:1) for 24 hours, followed by centrifugation to isolate the drug-loaded nanocomposite.
- Result: The loading efficiencies for NH2-MIL-125(Ti)@C1 and NH2-MIL-125(Ti)@C2 were determined to be 70.7% and 75.4%, respectively.
- Finding: The results indicate that the amine-functionalized nanocomposite significantly enhances the drug loading capacity compared to non-functionalized counterparts, demonstrating potential for improved therapeutic delivery.
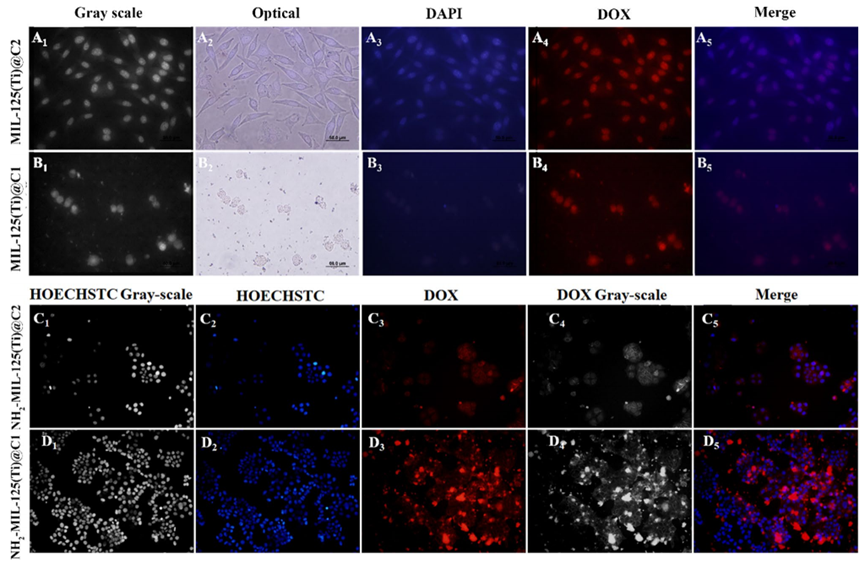
Figure 1. CLSM images demonstrating the DOX delivery efficiency of various nanocomposites on MCF-7 cells, reflecting the loading capacities of NH2-MIL-125(Ti)@C1 and NH2-MIL-125(Ti)@C2.
Experiment 3: Cellular Uptake and Transfection Efficiency
- Procedure: A549 lung cancer cells were exposed to the nanocomposite loaded with pCRISPR. The transfection efficiency was evaluated using fluorescence microscopy to assess the expression of GFP as a reporter gene.
- Result: The transfection efficiency reached 38.3% for NH2-MIL-125(Ti)@C1, while NH2-MIL-125(Ti)@C2 showed a lower efficiency of 16.6%.
- Finding: The enhanced transfection efficiency of NH2-MIL-125(Ti)@C1 suggests that the amine surface modification positively influences gene delivery, supporting the potential for effective gene therapy applications.
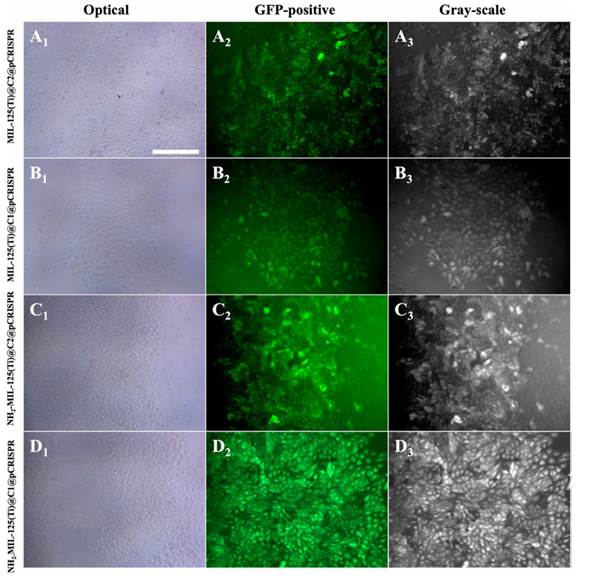
Figure 2. 2D fluorescence images illustrating the transfection efficacy of pCRISPR delivered by nanocomposites to A549 cells, with varying efficiencies observed in NH2-MIL-125(Ti)@C1 and NH2-MIL-125(Ti)@C2.
Experiment 4: Hemolysis Assay
- Procedure: The hemocompatibility of the nanocomposite was evaluated by incubating erythrocytes with varying concentrations of the nanocomposite and measuring the hemolysis rates.
- Result: The hemolysis rate for all tested compounds was found to be less than 1%.
- Finding: The low hemolysis rate indicates that the engineered nanocomposite possesses excellent biosafety, making it a promising candidate for in vivo applications in cancer treatment.
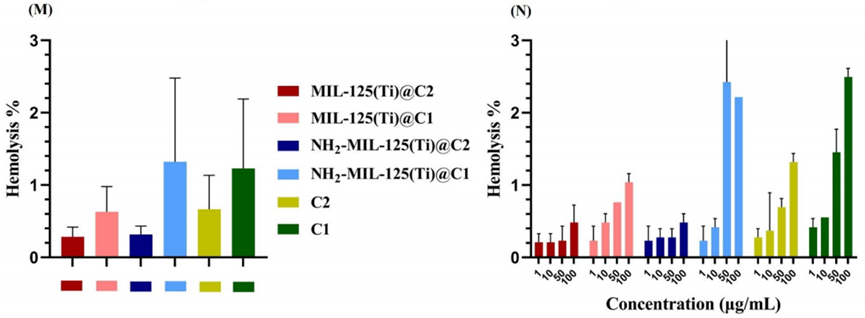
Figure 3. Hemolysis analysis showing the hemolytic percentages of the prepared compounds, indicating excellent biosafety with rates of less than 1%.
Key Findings and Implications for Cancer Treatment Strategies
This study successfully developed an innovative nanocomposite composed of amine-functionalized metal-organic frameworks (MIL-125(Ti)), poly(aniline-co-para-phenylenediamine), and manganese ferrite nanoparticles, aimed at enhancing the co-delivery of the chemotherapy drug doxorubicin (DOX) and the gene-editing tool pCRISPR to cancer cells. Key findings reveal that the engineered nanocomposite exhibited a remarkable drug loading efficiency of up to 75.4%, significantly higher than that of non-functionalized counterparts. The transfection efficiency reached an impressive 38.3% in A549 lung cancer cells, indicating that amine surface modification substantially improves cellular uptake and gene delivery.
Additionally, the study demonstrated excellent biosafety, with a hemolysis rate of less than 1%, suggesting that the nanocomposite is suitable for potential in vivo applications. The incorporation of a cell-imprinted substrate further optimized drug and gene delivery by mimicking the cellular microenvironment, thus enhancing therapeutic efficacy.
Reference:
Safarkhani, Moein, et al. “Engineered (NH2)-MIL-125 (Ti)/copolymer@ MnFe2O4 nanocomposite for synergistic eradication of cancer cells via DOX/pCRISPR delivery.” Advanced Composites and Hybrid Materials 7.1 (2024): 18.
