Editor: Nina
Scientists investigate the co-administration of liposomal doxorubicin and free quercetin to reduce doxorubicin-induced cardiotoxicity while maintaining its anti-cancer efficacy in a novel drug delivery approach.
Key Preview
Recent research conducted by a team of scientists from Shahid Sadoughi University of Medical Sciences reveals promising advancements in reducing the cardiotoxic effects associated with doxorubicin, a widely used chemotherapeutic agent. By co-administering liposomal doxorubicin and quercetin, the study demonstrates improved cardiac safety and efficacy, presenting a potential therapeutic strategy for cancer treatment.
Research Question
The primary research question addressed in this study is: How can the cardiotoxicity induced by doxorubicin be mitigated? The investigation specifically focuses on the effectiveness of combining liposomal doxorubicin with free quercetin to counteract cardiac damage.
Research Design and Strategy
The research was designed as a combination of in vitro and in vivo studies. Initially, the physicochemical properties of liposomal doxorubicin were characterized. Subsequently, the effects of various treatments, including empty liposomes, free doxorubicin, liposomal doxorubicin, and quercetin, were evaluated in animal models and on H9c2 cardiac cell lines.
Method
The team synthesized liposomal doxorubicin using the thin-film hydration method and assessed its properties such as size, encapsulation efficiency, and stability. The in vivo study involved administering treatments to male Wistar rats, followed by biochemical assessments of cardiac function and histopathological evaluations of heart tissues.
Key Results
The co-administration of liposomal doxorubicin and free quercetin resulted in significant reductions in cardiac enzyme levels associated with damage and oxidative stress. Key findings include decreased weight loss, lower levels of creatine kinase (CK-MB), lactate dehydrogenase (LDH), and malondialdehyde (MDA), alongside increased activity of antioxidant enzymes such as glutathione peroxidase and catalase.
Significance of the Research
This research highlights the potential of using liposomal formulations in conjunction with natural antioxidants like quercetin to enhance the safety profile of doxorubicin. The findings suggest a viable path for developing combination therapies that not only target cancer effectively but also minimize harmful side effects, ultimately improving patient outcomes.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, with its incidence expected to rise significantly due to factors such as population growth, aging, and lifestyle changes. The complexity of cancer biology, characterized by genetic mutations and heterogeneity among tumor cells, poses significant challenges in achieving effective treatment outcomes. Among the various chemotherapeutic agents, doxorubicin is widely employed for its potent anti-cancer properties, particularly in treating breast cancer, leukemia, and lymphoma. However, its clinical utility is severely limited due to dose-dependent cardiotoxicity, which can lead to long-term cardiac damage and compromise the overall health of patients.
Traditional drug delivery methods for doxorubicin primarily involve systemic administration through intravenous routes. This approach aims to maximize drug exposure to tumor cells while minimizing toxicity to healthy tissues. However, conventional delivery systems often result in a lack of specificity, leading to significant off-target effects, particularly on the heart. This non-selective distribution can cause severe side effects, including cardiomyopathy and heart failure, which ultimately restrict the dosage and frequency of doxorubicin administration. Consequently, the need for innovative strategies that enhance the therapeutic index of doxorubicin while mitigating its adverse effects has become increasingly urgent.
The distinct challenges associated with conventional doxorubicin therapy highlight the necessity for advanced drug delivery systems that can improve targeted drug delivery to tumor cells. One promising approach involves the use of nanotechnology to create lipid-based carriers, such as liposomes, which can encapsulate doxorubicin. These nanocarriers can provide controlled release profiles and reduce systemic toxicity through passive targeting mechanisms, allowing for preferential accumulation in tumor tissues due to the enhanced permeability and retention (EPR) effect. Additionally, incorporating natural antioxidants like quercetin into these formulations may further enhance their therapeutic potential by protecting against oxidative stress and reducing cardiotoxicity.
This research aims to investigate the efficacy of co-administering liposomal doxorubicin with free quercetin, focusing on minimizing the cardiotoxic effects associated with doxorubicin while maintaining its anti-cancer efficacy. Through the innovative use of liposomal formulations combined with natural compounds, this study seeks to establish a more effective and safer therapeutic strategy for cancer treatment, ultimately improving patient outcomes and quality of life.
Research Team and Aim
The research team responsible for this study was led by Dr. Hamidreza Dorostkar, who, along with his colleagues, conducted the research at Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The investigation took place from April to July 2023. The findings of this study were published in the paper titled “Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies” in the journal Pharmaceutics.
The aim of the research, as articulated by Dr. Dorostkar, was to develop a safer treatment approach for cancer patients receiving doxorubicin by utilizing liposomal formulations and the antioxidant properties of quercetin, focusing on reducing cardiotoxicity while maintaining therapeutic efficacy.
Experimental Process
Experiment 1: Synthesis of Liposomal Doxorubicin
Primary Technique: The primary technique employed in this study was the thin-film hydration method for synthesizing liposomal doxorubicin.
Key Steps:
- Preparation of Lipid Mixture: Soybean phosphatidylcholine (SPC), cholesterol, and distearoyl phosphoethanolamine-polyethylene glycol 2000 (DSPE-mPEG) were weighed in a molar ratio of 66.5:28.5:5.
- Solvent Evaporation: The lipid mixture was dissolved in chloroform and placed in a rotary evaporator to form a thin lipid film by evaporating the solvent under reduced pressure.
- Hydration: The lipid film was hydrated with a solution of doxorubicin (0.5 mg/mL) in phosphate-buffered saline (PBS; pH 7.4) at 55 °C for 60 minutes, allowing the drug to encapsulate within the liposomes.
- Sonication and Filtration: The liposomal suspension was sonicated to reduce vesicle size and then filtered through 0.45 µm and 0.22 µm filters to obtain uniform liposomes.
Data Collection and Analysis: The size, polydispersity index (PDI), and zeta potential of the synthesized liposomes were measured using dynamic laser scattering (DLS). The encapsulation efficiency (EE%) was calculated by lysing the liposomes with isopropanol and measuring absorbance via spectrophotometry.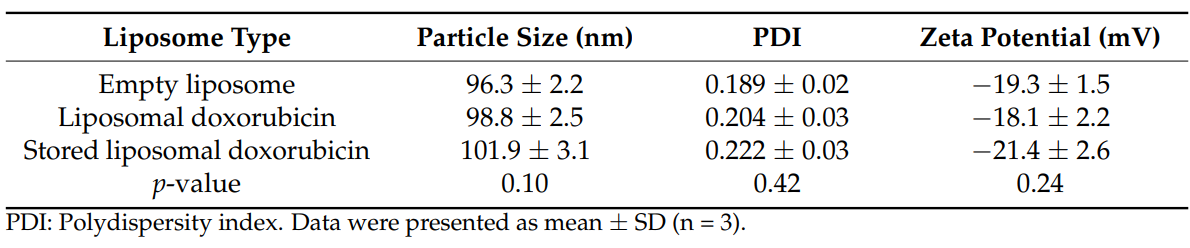
Table 1. Characterization of Liposomes
Result: The synthesized liposomal doxorubicin exhibited a mean size of 98.8 nm, a PDI of 0.204, and an EE% of approximately 85%. These characteristics indicate a successful encapsulation and appropriate size for biological applications.
Novel Aspect: This study utilized a simple thin-film hydration method, which offers advantages over traditional solvent evaporation methods by providing higher encapsulation efficiency and a more uniform size distribution in the synthesized liposomes.
Experiment 2: In Vitro Drug Release Evaluation
Primary Technique: In vitro drug release studies were conducted using a dialysis bag method to assess the release profiles of liposomal doxorubicin under varying pH conditions.
Key Steps:
- Preparation for Release Study: The dialysis bag (cut-off = 12-14 kDa) containing the liposomal doxorubicin was immersed in beakers filled with PBS at two different pH levels (7.4 and 5.4) at 37 °C and 42 °C.
- Sampling: At predetermined time intervals (1, 2, 4, 8, 24, 48, and 72 hours), samples were taken from the surrounding medium, and fresh PBS was added to maintain sink conditions.
- Measurement of Drug Concentration: The concentration of doxorubicin released was measured using spectrophotometric analysis according to a standard curve.
Data Collection and Analysis: The cumulative release was plotted against time, and the release kinetics were analyzed to determine the release profile and pH sensitivity of the liposomal doxorubicin.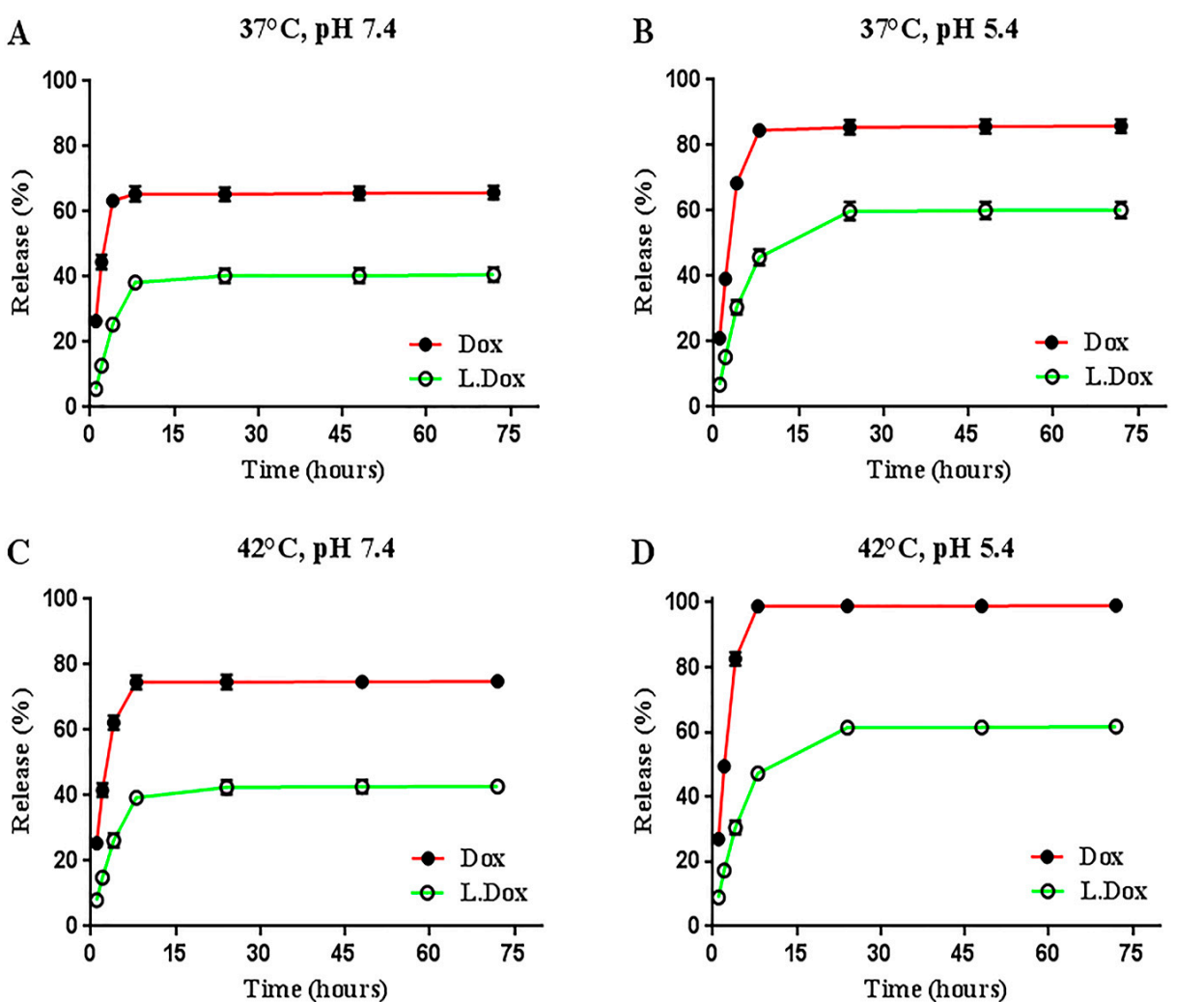
Figure 1. (A–D) Curves, cumulative release of free and liposomal doxorubicin in different conditions, at temperatures of 37 and 42 degrees Celsius and with pHs of 7.4 and 5.2 after 72 h. Data were presented as mean ± SD (n = 3). Dox: free doxorubicin, L.Dox: liposomal doxorubicin.
Result: The results indicated a controlled release pattern, with significant increases in drug release at pH 5.4 compared to pH 7.4, highlighting the pH sensitivity of the liposomes.
Novel Aspect: This release study demonstrated the liposomes’ ability to enhance drug accumulation in acidic tumor environments, a significant improvement over conventional drug delivery systems which do not exhibit such targeted release properties.
Experiment 3: In Vivo Animal Study
Primary Technique: The in vivo efficacy and safety of liposomal doxorubicin with quercetin were assessed using a male Wistar rat model.
Key Steps:
- Animal Grouping and Treatment: Forty-two male Wistar rats were divided into seven groups, receiving different treatments, including normal saline, quercetin, free doxorubicin, liposomal doxorubicin, and combinations thereof, for 12 days.
- Weight Monitoring: The body weight of the rats was measured on the first and last days of treatment to evaluate any weight changes due to the treatments.
- Sample Collection: On the final day, blood samples were collected for biochemical analysis, and heart tissues were harvested for further assessments.
Data Collection and Analysis: Serum levels of cardiac enzymes (CK-MB and LDH) were measured using commercial kits, and oxidative stress markers (MDA) were quantified to evaluate cardiac damage.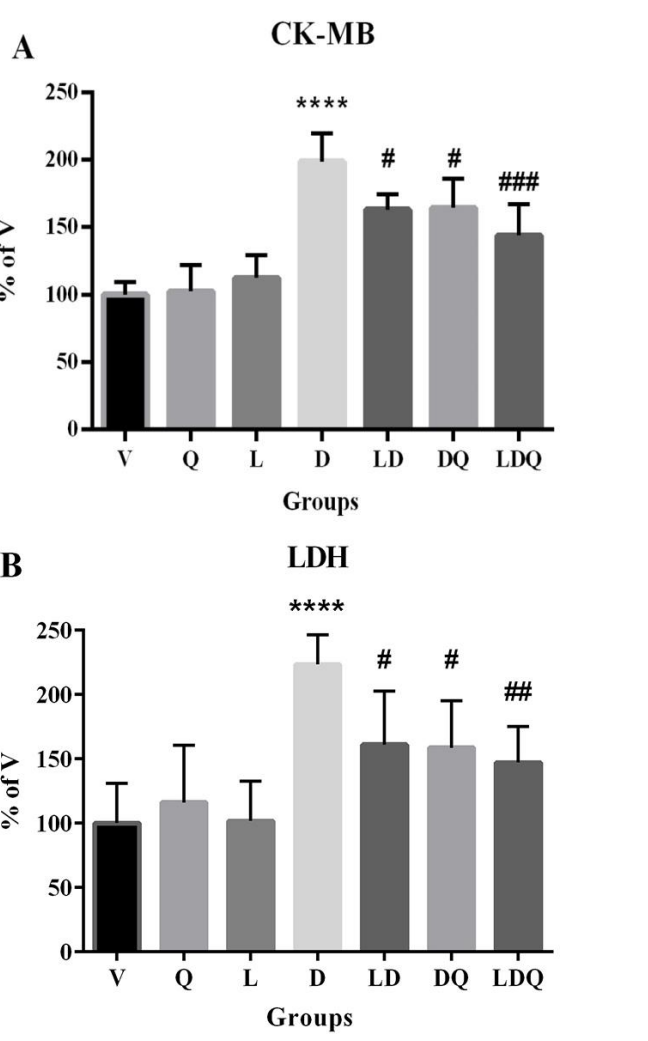
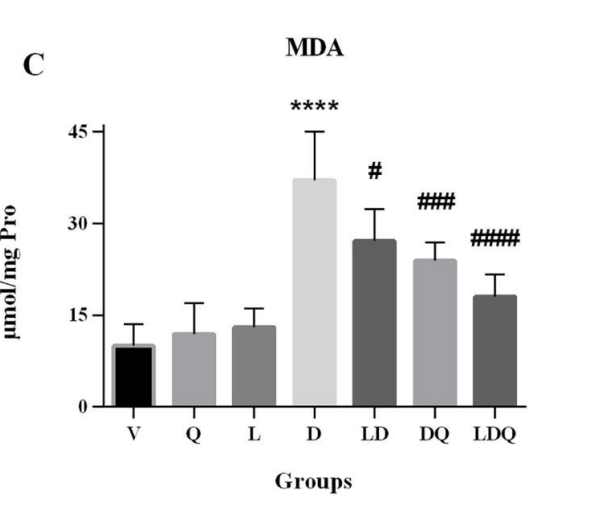
Figure 2. (A,B) Serum CK-MB and LDH levels compared to the vehicle group(%)©MDA content in different group is expressed as micromoles per milligram of protein.
Result: Co-administration of liposomal doxorubicin and quercetin significantly reduced CK-MB, LDH, and MDA levels, while increasing the activity of antioxidant enzymes, indicating improved cardiac safety and reduced toxicity.
Novel Aspect: The combination of liposomal doxorubicin and quercetin in an in vivo model represents a novel therapeutic strategy that not only targets cancer cells effectively but also protects cardiac tissue from chemotherapy-induced damage.
Experiment 4: Histopathological Evaluation
Primary Technique: Histopathological evaluations were conducted to assess tissue damage in cardiac samples using hematoxylin-eosin (HE) staining.
Key Steps:
- Tissue Preparation: Cardiac tissues were fixed in 10% formalin, embedded in paraffin, and sectioned at 5 µm thickness.
- Staining: The tissue sections were stained using HE staining to visualize the cellular architecture and identify any pathological alterations.
- Microscopic Examination: Stained sections were examined under a light microscope to evaluate the extent of myocardial injury.
Data Collection and Analysis: Histopathological changes were qualitatively analyzed, focusing on myocyte integrity, hypertrophy, and the presence of apoptosis or necrosis.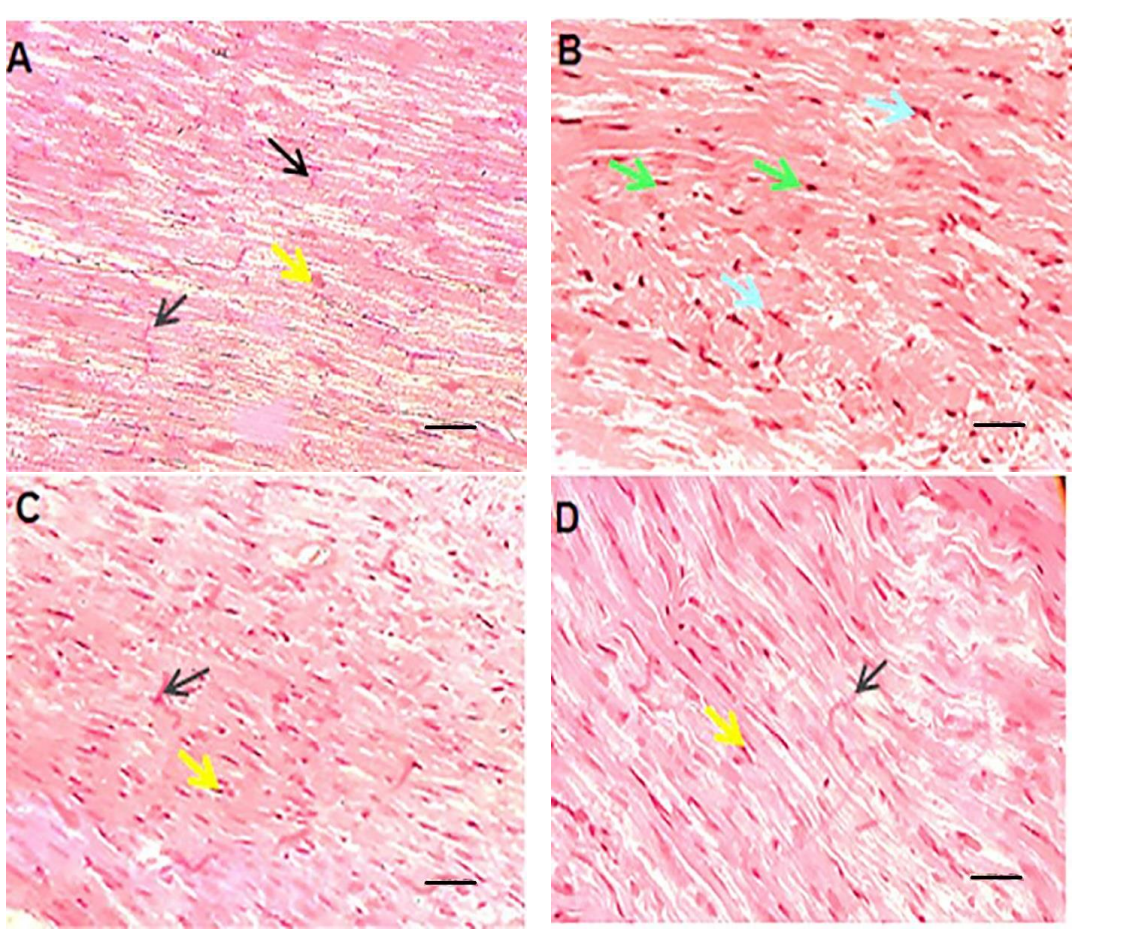
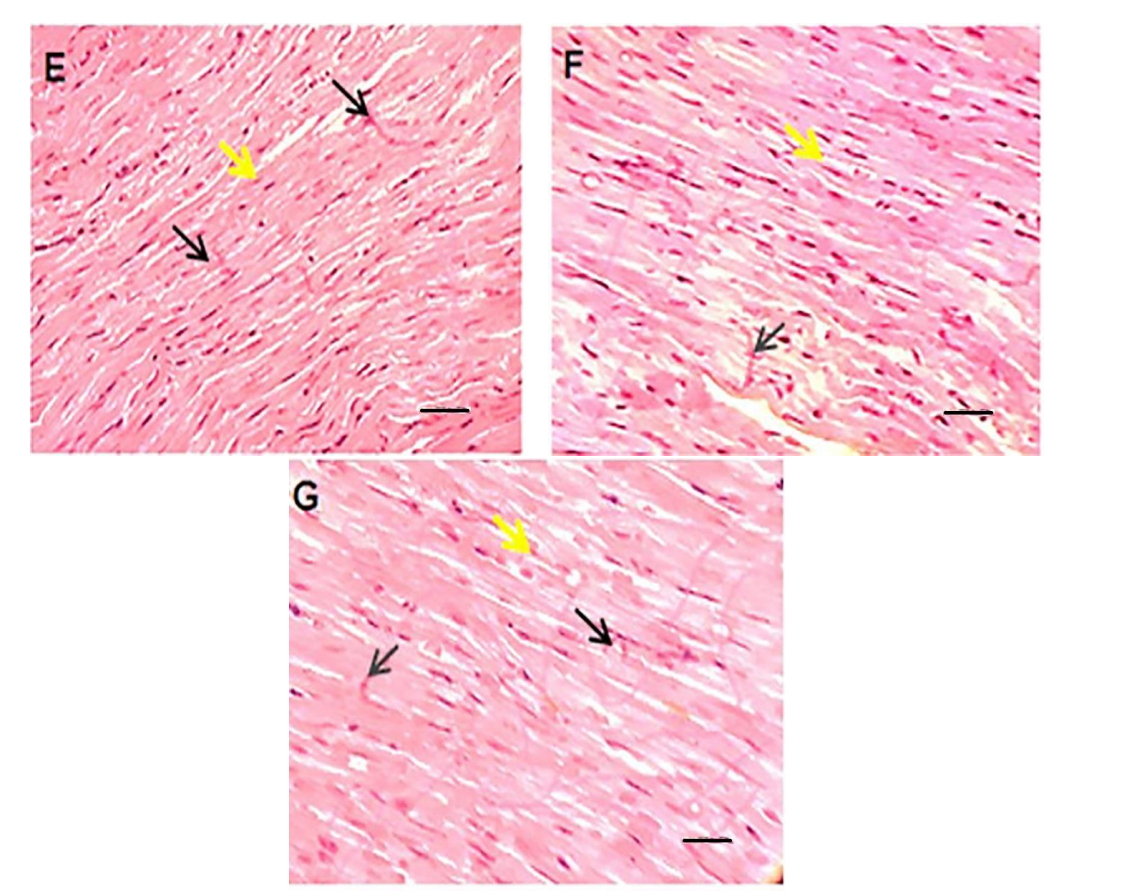
Figure 3. HE staining of left ventricular tissue of intervened-on rats in different groups (scale bar: 100 μm). In the vehicle or control group.
Result: The findings indicated that liposomal doxorubicin and quercetin co-administration resulted in less structural damage and improved histological appearance compared to free doxorubicin-treated groups.
Novel Aspect: This histopathological assessment provided critical insights into the protective effects of quercetin in conjunction with liposomal doxorubicin, showcasing a promising approach to minimize the adverse effects of chemotherapeutics on cardiac tissues.
Conclusion
The successful development of the drug delivery system presented in this study is achieved through the innovative combination of liposomal formulations and the natural antioxidant quercetin. By employing a thin-film hydration method, the researchers synthesized liposomal doxorubicin with favorable physicochemical properties, including appropriate size, high encapsulation efficiency, and pH sensitivity. This strategic approach not only enhances the targeted delivery of doxorubicin to cancer cells but also mitigates its cardiotoxic effects, addressing a critical limitation of traditional chemotherapy.
The highlights of the study include the significant reduction of cardiac enzyme levels associated with damage and oxidative stress in the presence of co-administered liposomal doxorubicin and quercetin. Furthermore, the research demonstrated improved cardiac safety, as evidenced by biochemical assessments and histopathological evaluations, which indicated less structural damage in cardiac tissues compared to free doxorubicin-treated groups. Overall, this research underscores the potential of utilizing advanced drug delivery systems to enhance therapeutic outcomes in cancer treatment while minimizing adverse effects, ultimately paving the way for improved patient care and quality of life.
Reference
Dorostkar, Hamidreza, et al. “Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies.” Pharmaceutics, vol. 15, no. 7, 2023, article 1920. MDPI, https://doi.org/10.3390/pharmaceutics15071920.
