Editor: Nina
Scientists develop a fibronectin-targeting and MMP-9-activatable imaging probe, CREKA-GK8-QC, to enhance fluorescence imaging and guide precise surgical resection of breast cancer, improving tumor detection and patient outcomes.
Key Preview
- Research Question: The study investigates the efficacy of a novel imaging probe, CREKA-GK8-QC, designed to target fibronectin and be activated by matrix metalloproteinase-9 (MMP-9) for fluorescence imaging and image-guided surgery of breast cancer. The research addresses the challenge of identifying residual cancer cells during surgery and detecting lung micro-metastases.
- Research Design and Strategy: The team employed a preclinical experimental design utilizing mouse models to evaluate the imaging probe’s effectiveness in detecting breast cancer and its metastatic lesions.
- Method: The study utilized in vivo fluorescence imaging, ex vivo histological analysis, and various biochemical assays to assess the probe’s targeting capabilities and its impact on surgical outcomes.
- Key Results: The CREKA-GK8-QC probe demonstrated a high specificity for targeting breast tumors, allowing for the detection of lesions as small as 1 mm with a tumor-to-background ratio of 1.53-fold higher than the control probe. The fluorescence image-guided surgery led to a significant reduction in residual tumors, improving survival rates from 0% to 80% in the experimental group.
- Significance of the Research: This research represents a significant advancement in breast cancer surgery, providing a tool that enhances the accuracy of tumor resection and reduces recurrence rates, potentially leading to better patient outcomes.
Introduction
Breast cancer is one of the most prevalent cancers diagnosed among women globally, accounting for significant morbidity and mortality rates. It arises from the uncontrolled growth of breast cells and can manifest in various forms, with triple-negative breast cancer (TNBC) representing one of the most aggressive subtypes. Despite advancements in early detection and treatment options, breast cancer remains a leading cause of cancer-related deaths, highlighting the urgent need for effective therapeutic strategies.
Traditional treatments for breast cancer primarily include surgery, chemotherapy, radiation therapy, and targeted therapies. A common strategy for drug delivery in these treatments involves systemic administration of chemotherapeutic agents, which circulate throughout the body to reach tumor sites. However, this conventional approach is often accompanied by significant challenges, including non-specific distribution of drugs, which can result in substantial toxicity to healthy tissues and organs. Such off-target effects frequently lead to adverse side effects, limiting the tolerability of treatment and necessitating dose reductions that can compromise therapeutic efficacy. Additionally, the presence of residual tumor cells post-surgery can lead to recurrence, further complicating patient outcomes.
The challenges associated with traditional drug delivery methods underscore the need for innovative strategies that can enhance the specificity and efficacy of cancer therapies. One promising approach involves the development of multifunctional imaging probes that target specific biomarkers associated with cancerous tissues. By combining therapeutic agents with imaging capabilities, these novel probes can facilitate real-time monitoring and precise localization of tumors, enabling targeted drug delivery while minimizing systemic toxicity. This innovative drug delivery strategy aims to improve treatment outcomes for breast cancer patients by ensuring that therapeutic agents are delivered selectively to malignant cells, thereby reducing the risk of recurrence and enhancing overall survival rates.
Research Team and Aim
The research was conducted by a multidisciplinary team of experts led by Zhongquan Cheng, who spearheaded the study at the Institute of Automation, Chinese Academy of Sciences. The research was carried out in 2023 and culminated in the publication of the paper titled “Fibronectin-targeting and metalloproteinase-activatable smart imaging probe for fluorescence imaging and image-guided surgery of breast cancer.” This work was published in the Journal of Nanobiotechnology.
The aim of the research, as articulated by the lead researcher, Zhongquan Cheng, was to “develop a novel imaging strategy that could enhance surgical outcomes by enabling real-time visualization of tumor margins, thereby improving the accuracy of tumor resection and reducing the risk of residual tumors in patients undergoing surgery for breast cancer.”
Experimental Process
Experiment 1: Synthesis of the CREKA-GK8-QC Imaging Probe
Primary Technique: The main method utilized in this study was the synthetic chemistry of a dual-targeted imaging probe, specifically designed to target fibronectin and be activated by matrix metalloproteinase-9 (MMP-9).
Key Steps:
- Preparation of Reactants: QSY21-NHS and DDE-GK8 were dissolved in dimethylformamide (DMF) at a molar ratio of 1:1.2.
- Reaction Conditions: TEA was added to the mixture, and the reaction was conducted under nitrogen protection for 24 hours at 40°C.
- Purification: The synthesized QSY21-GK8-DDE was purified through dialysis against deionized water.
- Removal of Protecting Groups: The DDE protection was subsequently removed using 2% hydrazine hydrate in dichloromethane (DCM) for 30 minutes.
- Final Conjugation: The purified product was then reacted with Cy5.5-NHS in DMF, followed by further purification to yield the final CREKA-GK8-QC probe.
Data Collection and Analysis: The synthesis was characterized using high-resolution mass spectrometry (HR-MS) to confirm the successful formation of the probe and its purity.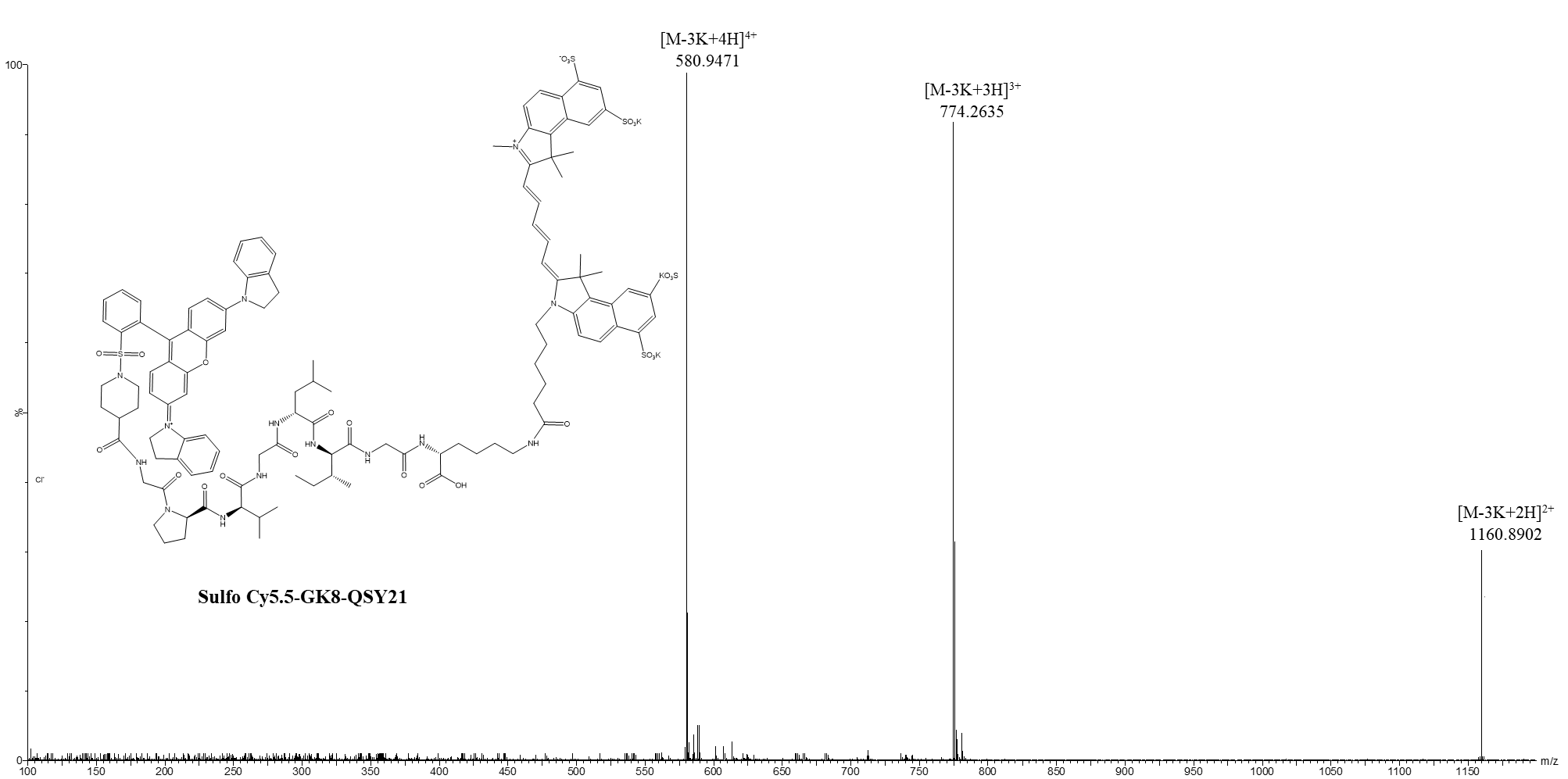
Figure 1. HR-MS spectra of Sulfo Cy5.5-GK8-QSY21
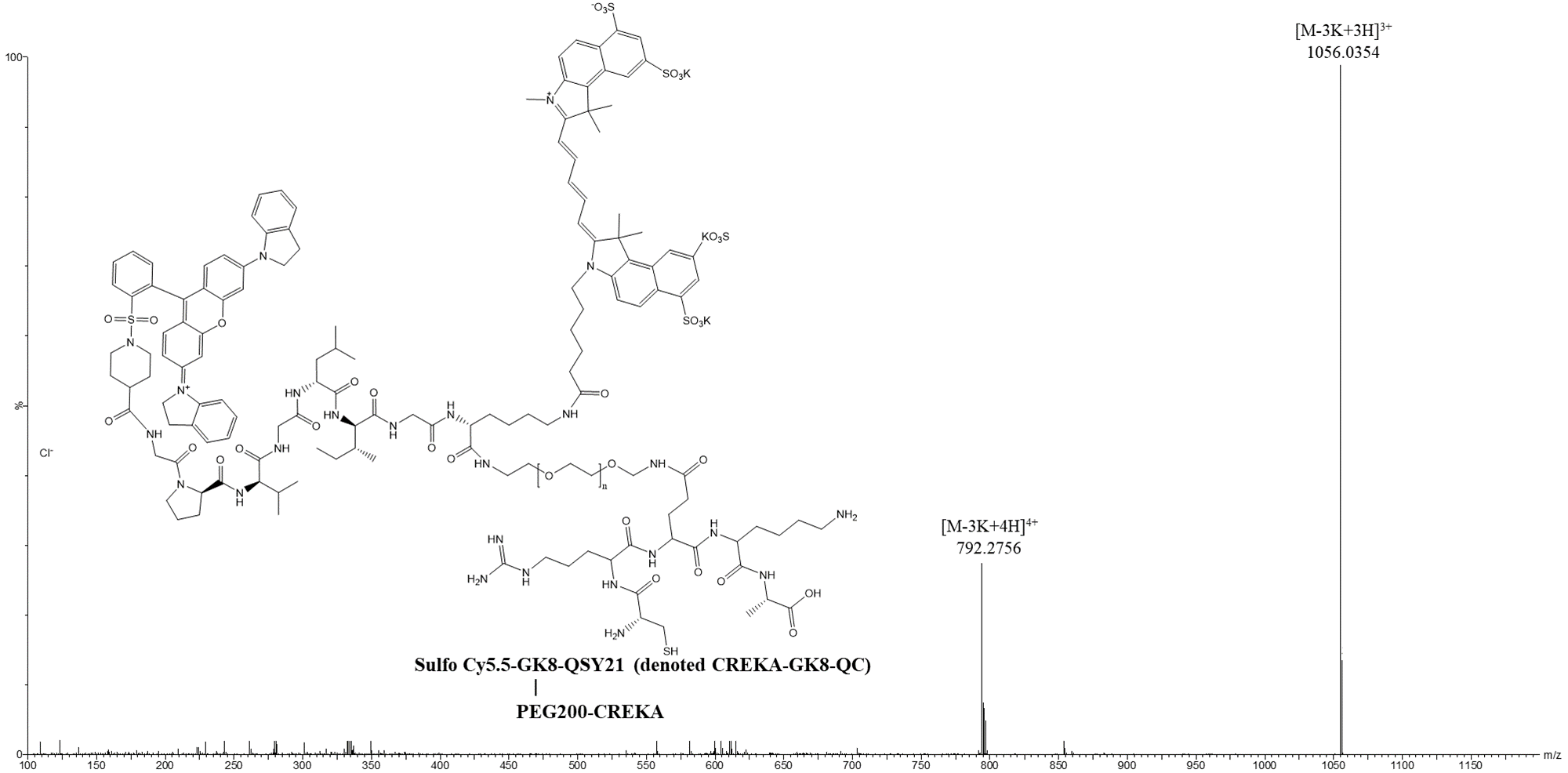
Figure 2. HR-MS spectra of CREKA-GK8-QC
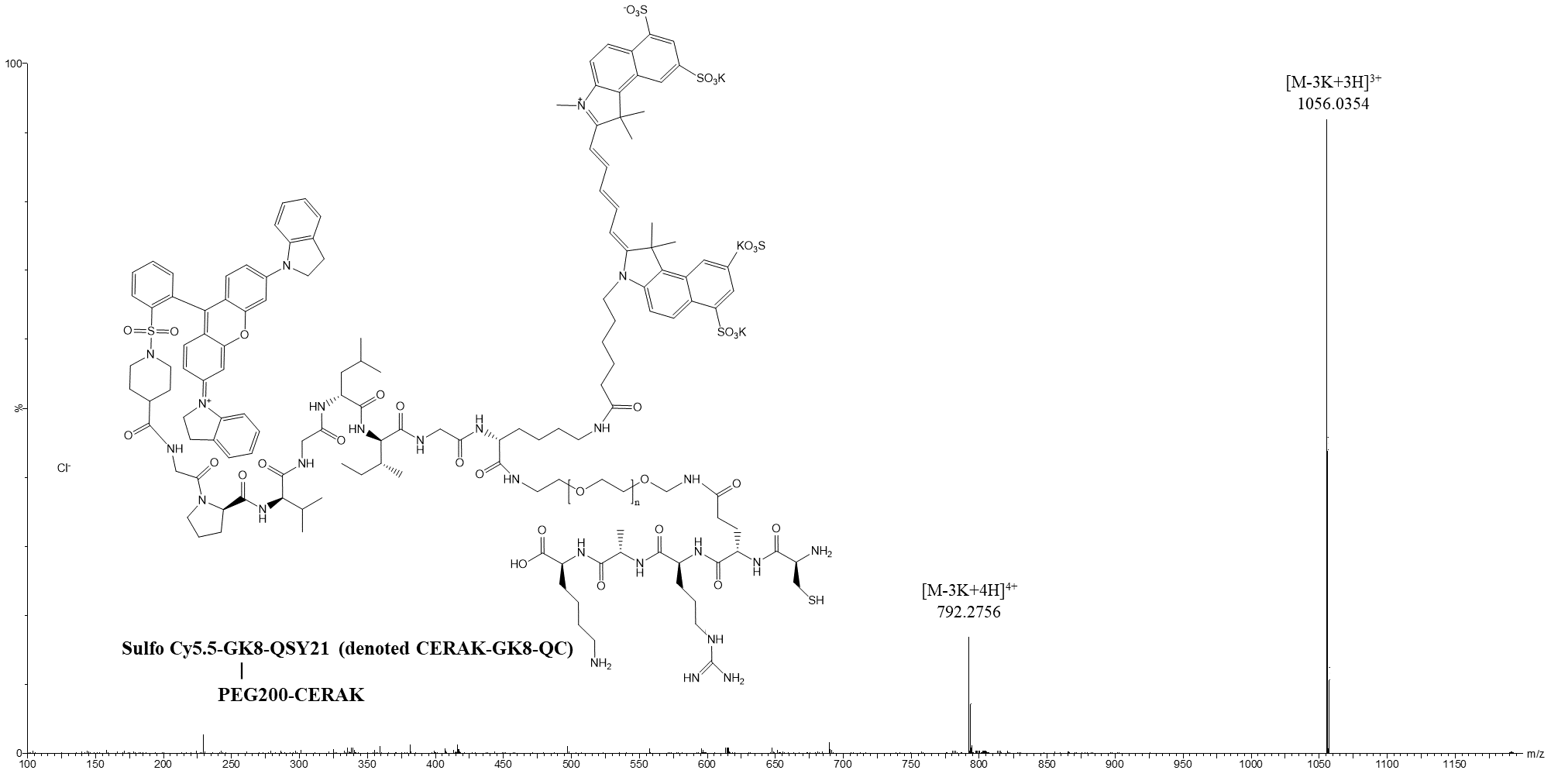
Figure 3. HR-MS spectra of CERAK-GK8-QC.
Result: The final product, CREKA-GK8-QC, was successfully synthesized as confirmed by HR-MS analysis, with an average diameter of 21.7 ± 2.5 nm, indicating the formation of uniform nanoparticles.
Novel Aspects: This synthesis approach allows for the creation of a dual-targeted imaging probe that integrates MMP-9 activation and fibronectin targeting, addressing the limitations of traditional nano-delivery systems by enhancing specificity and reducing off-target effects.
Experiment 2: In Vitro MMP-9 Responsiveness and Targeting Specificity
Primary Technique: The in vitro responsiveness of the CREKA-GK8-QC probe to MMP-9 was assessed using fluorescence imaging.
Key Steps:
- Preparation of Samples: Various concentrations of recombinant MMP-9 were prepared (0, 25, 50, 100, 200, and 400 ng/mL).
- Incubation: The probe CREKA-GK8-QC (5 μM) was incubated with MMP-9 in a 96-well plate for 2 hours at 37°C.
- Inhibition Test: For a competitive assay, MMP-9 was pre-incubated with GM6001 (150 μM) before adding the CREKA-GK8-QC probe.
Data Collection and Analysis: Fluorescence intensity was measured using an IVIS Lumina System, and the data were analyzed to determine the correlation between MMP-9 concentration and fluorescence response.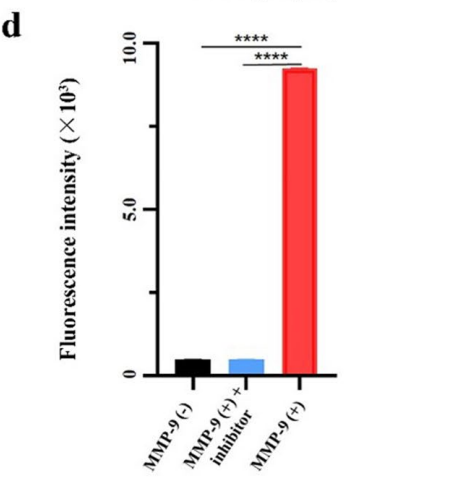
Figure 4. Fluorescence intensity of CREKA-GK8-QC treated with 0 ng/mL, 400 ng/mL, and combined 150 μM MMP-9 inhibitor and 400 ng/mL of MMP-9 protein using the ultraviolet–visible spectrophotometer, ****p < 0.0001.
Result: The probe exhibited a significant increase in fluorescence intensity correlating with MMP-9 concentration, with an R² value of 0.97, demonstrating a robust responsiveness. The competitive assay showed a marked reduction in fluorescence when GM6001 was present, confirming the specificity of the probe.
Novel Aspects: The dual-targeting mechanism of CREKA-GK8-QC offers a significant advantage over traditional probes, as it allows for precise detection of enzymatic activity in real-time, thus enhancing the reliability of tumor imaging.
Experiment 3: In Vivo Fluorescence Imaging of Tumors
Primary Technique: In vivo fluorescence imaging (FMI) was utilized to evaluate the targeting capabilities of the CREKA-GK8-QC probe in a murine model of breast cancer.
Key Steps:
- Animal Preparation: 4T1 breast carcinoma cells were implanted in the right mammary fat pad of BALB/c mice to establish orthotopic tumors.
- Probe Administration: Once the tumors reached approximately 100 mm³, mice were intravenously injected with CREKA-GK8-QC (100 μg/mL).
- Imaging Schedule: NIR fluorescence images were obtained at various time points (0, 30 min, 1, 2, 4, 12, and 48 hours post-injection).
Data Collection and Analysis: The fluorescence signals were quantified and tumor-to-background ratios (TBR) were calculated to assess the probe’s localization and retention in the tumors.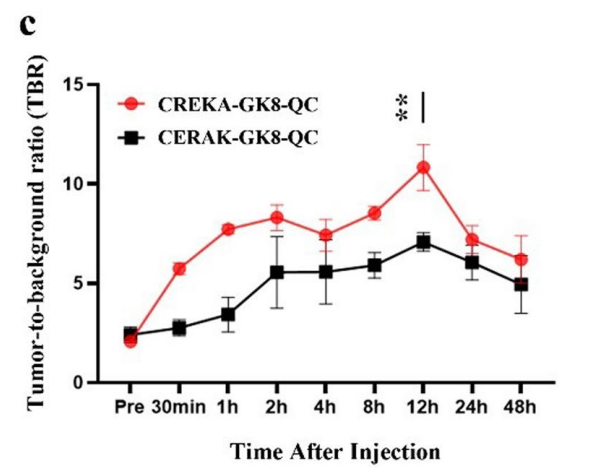
Figure 5. Quantifcation of in vivo fuorescence imaging of tumor to background ratio (TBR). Data are represented as the mean ± SD, n = 3, **p < 0.01.
Result: The CREKA-GK8-QC probe demonstrated significant tumor localization with a peak TBR at 12 hours, approximately 1.53-fold higher than the control probe, indicating superior targeting efficiency.
Novel Aspects: This study highlights the capability of the CREKA-GK8-QC probe to provide real-time imaging of tumors, which is a marked improvement over conventional imaging methods that often lack specificity and sensitivity.
Experiment 4: Fluorescence Image-Guided Surgery
Primary Technique: The effectiveness of the CREKA-GK8-QC probe for image-guided surgical resection of breast tumors was evaluated.
Key Steps:
- Group Assignment: Mice bearing orthotopic 4T1 tumors were randomly assigned to two groups: fluorescence-guided surgery and conventional surgery.
- Intraoperative Imaging: Real-time fluorescence imaging was performed using a fluorescence stereomicroscope to guide surgical resection.
- Surgical Procedure: Tumor resection was carried out while monitoring fluorescence signals to identify tumor margins.
Data Collection and Analysis: Post-surgical residual tumors were assessed using bioluminescent imaging (BLI) and histological analysis to quantify the extent of tumor removal.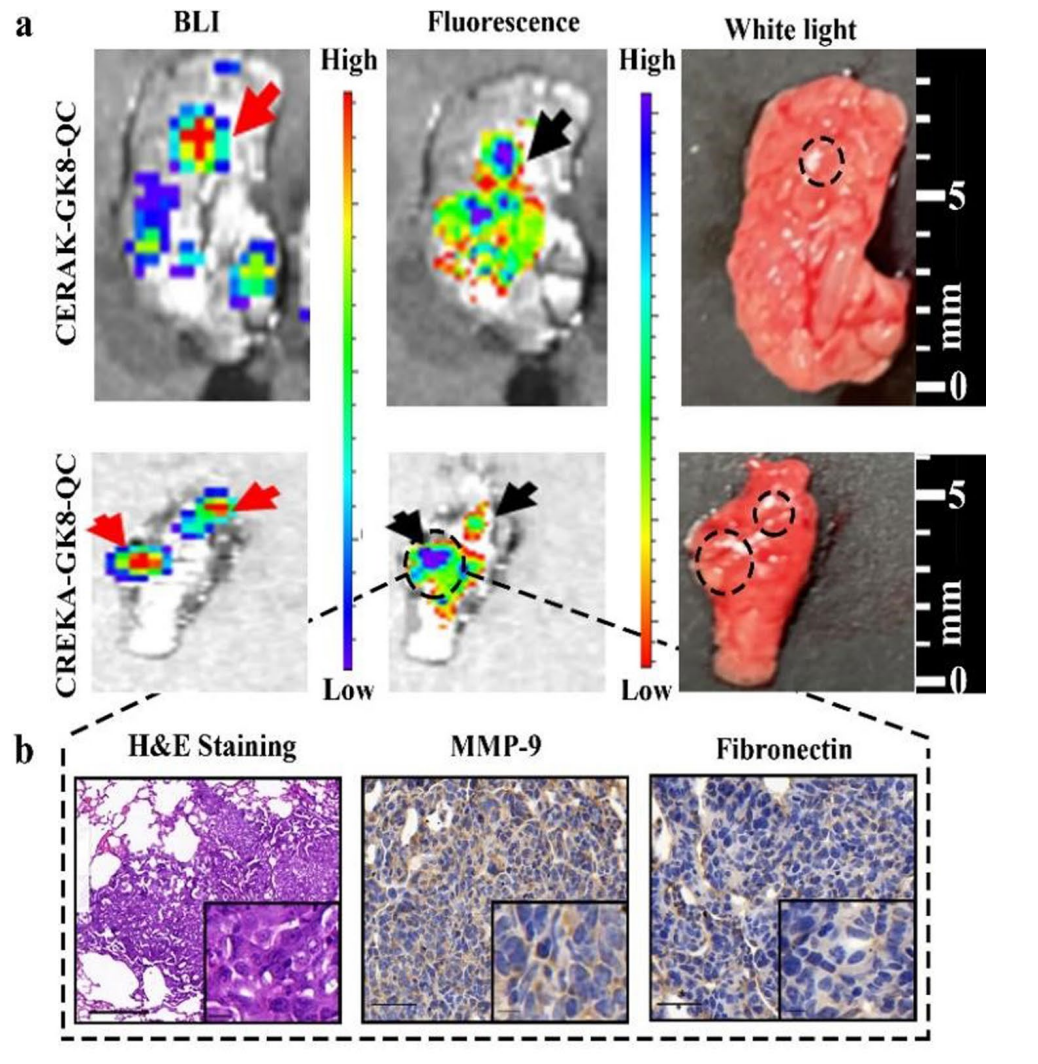
Figure 6. a. Ex vivo BLI images, fuorescence images, and white light images of metastatic lungs at 24 h post-injection of CREKA-GK8-QC or CERAK-GK8-QC. The red and black arrows indicated the lightened-up metastatic lesions of lungs. b. H&E and immunohistochemical staining was utilized to confrm the true-positive metastatic lesion. Scale bar 50 µm. Inset: enlarged images of the metastasis lesions (scale bar 10 µm).
Result: The fluorescence image-guided surgery resulted in complete tumor resection with no detectable residual tumors, while the conventional surgery group exhibited significant tumor remnants, leading to a recurrence rate of 100%.
Novel Aspects: This experiment underscores the practical application of the CREKA-GK8-QC probe in the surgical setting, enhancing precision in tumor resection and minimizing the risk of recurrence, which is a critical advancement over standard surgical approaches.
Experiment 5: Detection of Lung Micro-Metastases
Primary Technique: Ex vivo fluorescence imaging was employed to assess the probe’s ability to detect lung micro-metastases following systemic administration.
Key Steps:
- Establishment of Lung Metastasis Model: 4T1 cells were injected into the tail vein of mice to create lung metastatic lesions.
- Probe Administration: Mice were injected with CREKA-GK8-QC, and fluorescence imaging was conducted at various time points.
- Ex Vivo Imaging: After euthanasia, lung tissues were collected and subjected to fluorescence analysis to identify metastatic lesions.
Data Collection and Analysis: The fluorescence intensity in lung tissues was quantified, and the detection rate of metastatic lesions was calculated based on the imaging results.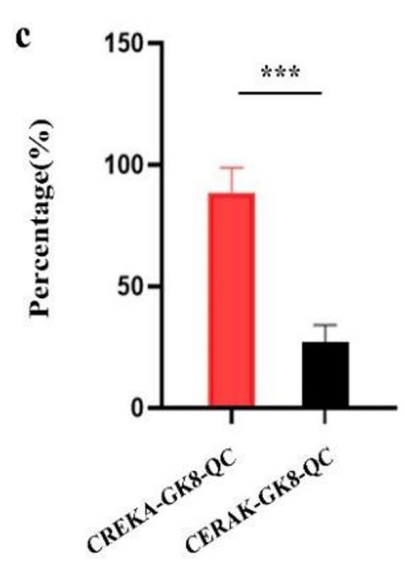
Figure 7. Analysis of percentage of detection rate of metastatic lesions by CREKA-GK8-QC or CERAK-GKE-QC, respectively. Data represented as the mean ± SD, n = 3, ***p < 0.001
Result: The CREKA-GK8-QC probe detected 84.6% of metastatic lesions, significantly higher than the control probe, which detected only 25%, indicating superior sensitivity.
Novel Aspects: This ability to identify micro-metastases as small as 1 mm represents a substantial advancement over traditional imaging techniques, which often fail to detect such small lesions in real-time surgical settings.
Conclusion
The successful development of the CREKA-GK8-QC imaging probe as a drug delivery system was achieved through a meticulously designed synthesis process that integrated dual-targeting capabilities for both fibronectin and matrix metalloproteinase-9 (MMP-9). This innovative probe demonstrated high specificity for tumor tissues and robust responsiveness to enzymatic activation, enabling precise fluorescence imaging and facilitating accurate tumor localization during surgical procedures.
The highlights of the study include the probe’s ability to detect primary breast tumors and micro-metastatic lesions with high sensitivity, as well as its significant impact on surgical outcomes. Notably, fluorescence image-guided surgery using the CREKA-GK8-QC probe resulted in complete tumor resection with no detectable residuals, leading to a marked improvement in survival rates among the treated mice. This research underscores the potential of advanced imaging probes to enhance the precision of cancer surgeries while reducing recurrence rates, paving the way for future clinical applications in breast cancer treatment.
Reference
Cheng, Zhongquan, et al. “Fibronectin-targeting and metalloproteinase-activatable smart imaging probe for fluorescence imaging and image-guided surgery of breast cancer.” Journal of Nanobiotechnology, vol. 21, no. 112, 2023, https://doi.org/10.1186/s12951-023-01868-5.
