Editor: Tiffany
Researchers have developed multifunctional nanoparticles that combine chemotherapy, phototherapy, oxygen generation, and immunotherapy, achieving an 89.20% tumor inhibition rate in preclinical breast cancer models.
Key Highlights
- Research Question:
How can the limitations of current breast cancer treatments, particularly tumor hypoxia in photodynamic therapy, be addressed? - Research Difficulties:
Tumor hypoxia reduces the efficacy of photodynamic therapy, and traditional chemotherapy faces issues like systemic toxicity and drug resistance. - Key Findings:
ZNIDL NPs demonstrated an 89.20% tumor inhibition rate, reduced metastasis, and induced immunogenic cell death in preclinical models. - Innovative Aspects:
The integration of oxygen generation, chemotherapy, phototherapy, and immunotherapy into a single nanoparticle system. - Importance of the Study:
This approach offers a potential strategy to enhance treatment efficacy, reduce side effects, and prevent recurrence in breast cancer.
Challenges in Breast Cancer Treatment: Hypoxia and Therapeutic Limitations
Breast cancer remains a major global health concern, characterized by high mortality rates due to its invasive properties. Common symptoms include a lump in the breast, changes in breast shape, skin dimpling, and, in advanced stages, bone pain, swollen lymph nodes, and jaundice. Current treatment options include surgery, chemotherapy, radiation, hormone therapy, and targeted therapies. Chemotherapy, often utilizing doxorubicin (DOX), is a standard approach but is limited by systemic toxicity and drug resistance. Phototherapy, encompassing photothermal therapy (PTT) and photodynamic therapy (PDT), provides a non-invasive alternative. However, PDT’s efficacy is reduced by tumor hypoxia, a condition of low oxygen levels that limits the generation of reactive oxygen species (ROS) essential for cancer cell destruction.
Developing Multifunctional Nanoparticles to Overcome Hypoxia and Enhance Therapy
The study sought to address limitations in breast cancer treatments, specifically the issue of tumor hypoxia in PDT. The primary research question was how to develop a multifunctional nanoparticle system that integrates oxygen generation, chemotherapy, phototherapy, and immunotherapy to achieve synergistic antitumor effects. The objectives included designing ZnO@DOX/ICG-LMHP nanoparticles (ZNIDL NPs) to alleviate hypoxia, deliver DOX for chemotherapy, employ indocyanine green (ICG) for PTT and PDT, and induce immunogenic cell death (ICD) for immunotherapy. This research, conducted by Xuan Zhang and colleagues from Peking University, was published in the Journal of Functional Biomaterials in 2024.
Synthesis, Characterization, and Preclinical Evaluation of ZNIDL Nanoparticles
The study employed a series of experimental procedures to synthesize and assess the ZNIDL NPs. Key methods and findings are outlined below.
1. Synthesis and Characterization of ZNIDL NPs
- Procedure: ZNIDL NPs were synthesized via a sol-gel method. Zinc oxide (ZnO) nanoparticles were functionalized with 3-aminopropyltrimethoxysilane to form ZnO-NH₂ NPs, then loaded with DOX and ICG, and coated with low-molecular-weight heparin (LMHP).
- Result: The nanoparticles measured 34.04 ± 0.31 nm in size with a zeta potential of -24.87 ± 0.06 mV. They exhibited pH-sensitive drug release, with greater release at pH 5.5 than at pH 7.4, alongside oxygen generation and photothermal effects under laser irradiation.
- Finding: The nanoparticles’ size and zeta potential support their stability and tumor penetration. The pH-sensitive release facilitates targeted drug delivery in the acidic tumor microenvironment, while oxygen generation mitigates hypoxia, potentially enhancing PDT.
2. In Vitro Antitumor Activity
- Procedure: The Sulforhodamine B (SRB) assay and apoptosis detection evaluated antitumor effects in 4T1 breast cancer cells treated with ZNIDL NPs and laser irradiation.
- Result: Cell death increased with concentration, peaking at 4 µM DOX equivalent. The apoptosis rate reached 38.90% in the ZNIDL NPs plus laser group, exceeding rates in other groups.
- Finding: The combined use of chemotherapy, PDT, and PTT in ZNIDL NPs enhances antitumor activity compared to single-modality treatments, demonstrating the value of integration.
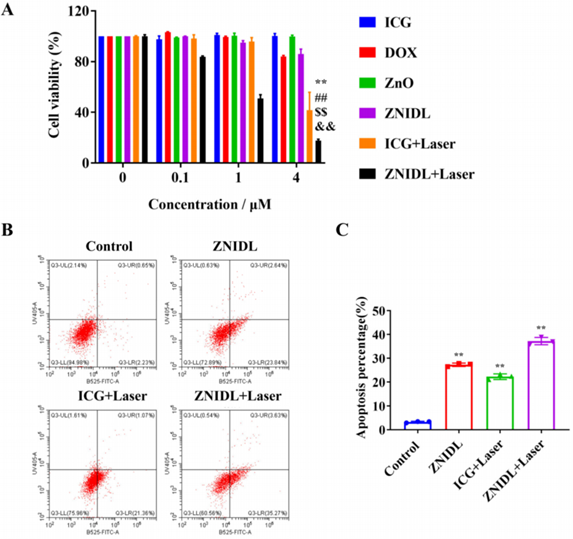
Figure 1. In vitro antitumor activity of ZNIDL NPs.
3. In Vivo Antitumor Efficacy
- Procedure: A 4T1 tumor-bearing BALB/c mouse model received peri-tumoral ZNIDL gel injections followed by laser irradiation. Tumor growth, metastasis, and survival were monitored.
- Result: The ZNIDL gel plus laser group achieved an 89.20% tumor growth inhibition rate, fewer pulmonary nodules, and improved survival. Histological analysis showed increased apoptosis and reduced hypoxia in treated tumors.
- Finding: The nanoparticles effectively inhibit primary tumor growth and metastasis, indicating potential for comprehensive cancer treatment.
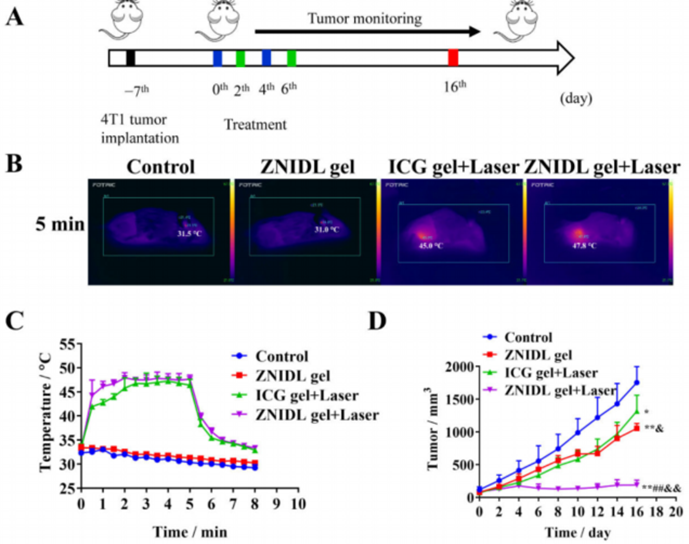
Figure 2. In vivo antitumor efficacy in 4T1 tumor-bearing mice.
4. Evaluation of Immunogenic Cell Death (ICD) and Immune Response
- Procedure: ICD was assessed via calreticulin (CRT) exposure, ATP release, and HMGB1 release. Immune activation was measured using flow cytometry and immunofluorescence.
- Result: ZNIDL NPs elevated ICD markers, promoting dendritic cell maturation and T cell infiltration in tumors and lymph nodes.
- Finding: ICD induction and immune activation contribute an additional antitumor mechanism, relevant for reducing metastasis and recurrence.
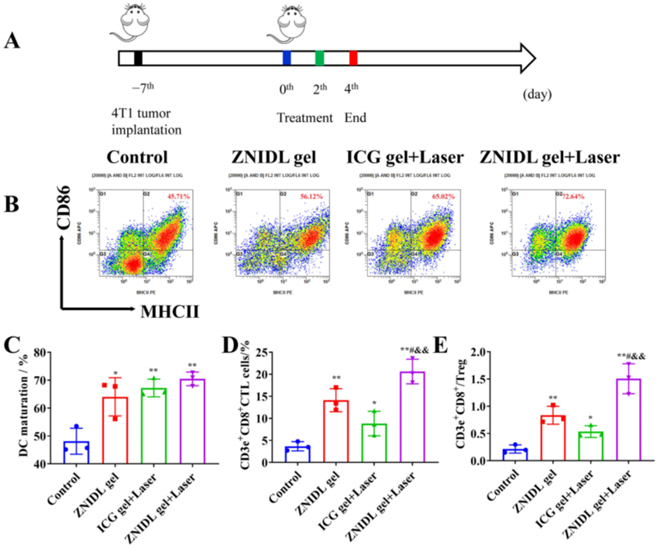
Figure 3. Immune response induced by ZNIDL NPs.
Integrated Therapeutic Approach Shows Promise for Improved Breast Cancer Outcomes
The study introduces ZNIDL NPs as a multifunctional system integrating oxygen generation, chemotherapy, phototherapy, and immunotherapy for breast cancer treatment. The nanoparticles’ oxygen production addresses tumor hypoxia, enhancing PDT efficacy. DOX delivery supports chemotherapy, while ICG enables PTT and PDT for direct tumor cell targeting. ICD induction further activates an antitumor immune response, offering potential long-term effects. Key results include an 89.20% tumor inhibition rate and reduced metastasis in preclinical models. This approach advances treatment efficacy by combining multiple modalities into a single nanoparticle system, contributing to the field through its targeted and integrated strategy.
Reference:
Li, Zhuoyue, et al. “Multifunctional ZnO@ DOX/ICG-LMHP nanoparticles for synergistic multimodal antitumor activity.” Journal of Functional Biomaterials 15.2 (2024): 35.
