Editor: Nina
Scientists develop retinoic acid-modified galangin nanoparticles for targeted delivery to hepatic stellate cells, enhancing the anti-fibrotic effects in the treatment of hepatic fibrosis.
Key Preview
- Research Question: The study investigates the efficacy of galangin delivered by retinoic acid-modified nanoparticles in targeting hepatic stellate cells (HSC) for treating hepatic fibrosis (HF).
- Research Design and Strategy: The research employs a nanoparticle-based drug delivery system to enhance the bioavailability and specificity of galangin, a flavonoid with potential anti-fibrotic properties, aimed at HSC.
- Method: The nanoparticles were developed using acrylic resin and modified with retinoic acid to improve targeting and bioavailability. Various in vitro and in vivo pharmacodynamic studies were conducted to assess the effectiveness of the treatment.
- Key Results: The study found that the retinoic acid-modified galangin nanoparticles (RA-GA-NPs) significantly enhanced the anti-fibrotic effects of galangin, with a notable increase in bioavailability. The RA-GA-NPs demonstrated a 2.65-fold increase in serum concentration compared to free galangin.
- Significance of the Research: This research provides a promising new avenue for treating hepatic fibrosis, a condition currently lacking effective therapies, by utilizing advanced nanotechnology to enhance drug delivery and efficacy.
Introduction
Hepatic fibrosis (HF) is a chronic and progressive condition characterized by the excessive accumulation of extracellular matrix proteins in the liver, primarily as a response to various liver injuries such as viral hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease. If left untreated, HF can progress to more severe forms of liver disease, including cirrhosis and hepatocellular carcinoma, leading to significant morbidity and mortality. The pathophysiology of HF involves the activation of hepatic stellate cells (HSC), which transition from a quiescent state to a myofibroblast-like phenotype, resulting in increased production of collagen and other fibrogenic factors. As the disease advances, the liver’s architecture becomes increasingly disrupted, impairing its functional capacity.
Traditional drug delivery strategies for treating hepatic fibrosis typically involve the systemic administration of pharmacological agents. This approach often utilizes oral or injectable formulations that aim to reduce the activation of HSC and promote their apoptosis. However, conventional treatment methods face several critical challenges, including low bioavailability of therapeutic agents, non-specific distribution, and rapid clearance from the bloodstream. These limitations can significantly diminish the therapeutic efficacy of the drugs, often resulting in suboptimal treatment outcomes and a lack of effective therapies for patients suffering from HF.
Given these challenges, there is a pressing need for innovative drug delivery strategies that enhance the efficacy and specificity of treatments for hepatic fibrosis. Recent advancements in nanotechnology have led to the development of nanoparticle-based drug delivery systems, which can improve the bioavailability and targeted delivery of therapeutic agents. By encapsulating drugs within nanoparticles that can specifically target HSC, these systems offer a promising solution to the limitations of traditional treatment approaches. For instance, the use of retinoic acid-modified nanoparticles has emerged as a potential strategy to enhance the targeting of HSC, providing a dual benefit of improved therapeutic delivery and reduced systemic side effects. This innovative approach not only aims to enhance the effectiveness of existing anti-fibrotic agents but also holds the potential to revolutionize the treatment landscape for hepatic fibrosis, paving the way for more effective therapeutic interventions.
Research Team and Aim
The research was conducted by a multidisciplinary team led by Yuanguo Xiong, along with Bing Wu and their colleagues from the Department of Pharmacy at Renmin Hospital of Wuhan University, along with other affiliated institutions. The study was carried out in 2023 and is titled “Galangin delivered by retinoic acid-modified nanoparticles targeted hepatic stellate cells for the treatment of hepatic fibrosis.” This work was published in the journal RSC Advances.
The primary aim of the research, as articulated by lead researcher Yuanguo Xiong, was to design an effective drug delivery system that enhances the anti-fibrotic effects of galangin while minimizing its limitations due to low bioavailability. The study specifically sought to develop retinoic acid-modified nanoparticles that would target hepatic stellate cells (HSC) in order to improve therapeutic outcomes for patients suffering from hepatic fibrosis.
Experimental Process
1. Preparation of Nanoparticles
Primary Technique:
The main method employed in this study is the nanoprecipitation technique, which is utilized to create galangin-loaded nanoparticles (GA-NPs) and retinoic acid-modified galangin nanoparticles (RA-GA-NPs) for targeted drug delivery.
Key Steps:
- Step 1: Dissolve 1 mg of galangin (GA) and 150 mg of Eudragit® RS100 (Eud RS100) in 2 mL of methanol to form the organic phase at room temperature.
- Step 2: Under gentle stirring, gradually add the organic phase to 4 mL of an aqueous phase containing deionized water and 1.0% (v/v) Tween-80.
- Step 3: Homogenize the mixture using ultrasound for 10 minutes, followed by continuous mechanical stirring for an additional 30 minutes to ensure thorough mixing.
- Step 4: Remove the excess organic solvent through rotary vacuum evaporation at 50 °C to obtain the GA-NP suspension.
Data Collection and Analysis:
The resulting nanoparticles were characterized for size, polydispersity index (PDI), and zeta potential using a Zetasizer ZS90 (Malvern Instruments). The drug loading capacity was assessed via high-performance liquid chromatography (HPLC).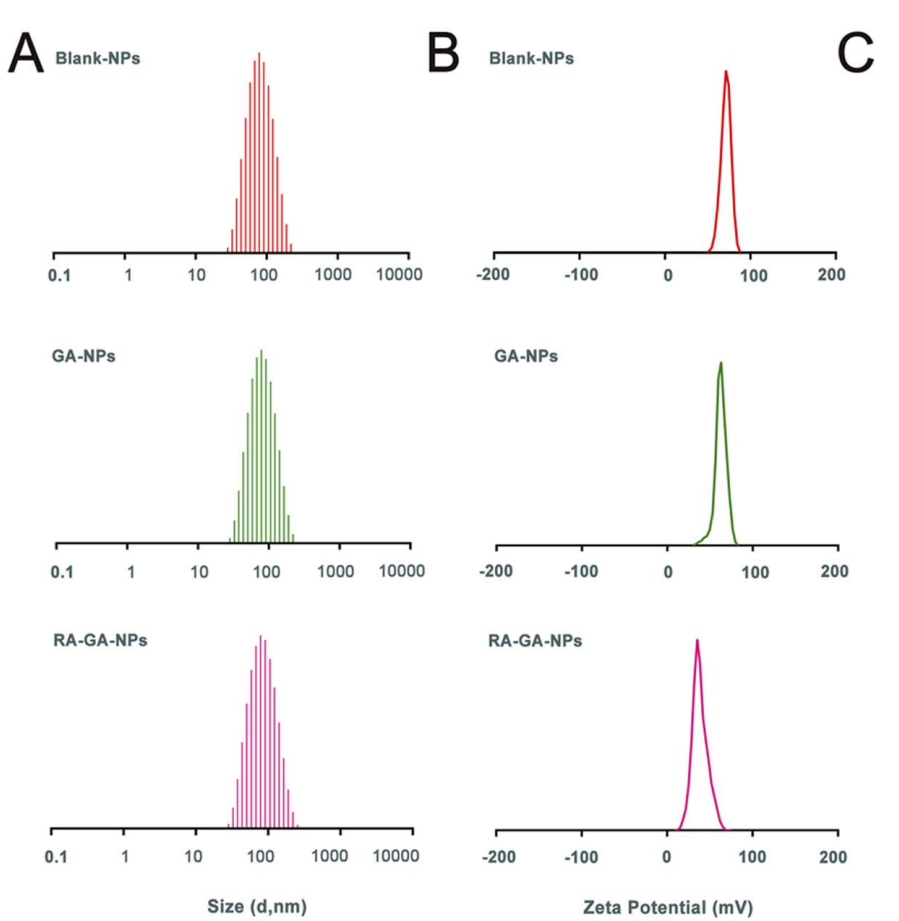
Figure 1. The physical characterization of nanoparticles. (A) the adequate particle size of nanoparticles; (B) the zeta potential of nanoparticles;
Result:
The GA-NPs exhibited a mean particle size of 71.66 ± 0.410 nm, a PDI of 0.187 ± 0.005, and a zeta potential of +65.1 ± 2.04 mV. The encapsulation efficiency was found to be 97.33 ± 1.02%.
Novel Aspect:
This study introduces a modified approach to nanoprecipitation that allows for the efficient encapsulation of hydrophobic compounds like galangin while ensuring high drug loading and stability, enhancing the bioavailability of the drug compared to traditional methods.
2. Surface Modification with Retinoic Acid
Primary Technique:
Surface modification of the nanoparticles was performed using a simple adsorption technique to create RA-GA-NPs.
Key Steps:
- Step 1: Dissolve 100 mg of retinoic acid (RA) in 1 mL of methanol.
- Step 2: Add the RA solution to 10 mL of the previously prepared GA-NP suspension and stir the mixture for 4 hours at room temperature.
- Step 3: Remove the organic solvent by rotary vacuum evaporation at 50 °C to obtain the RA-GA-NP suspension.
Data Collection and Analysis:
The modified nanoparticles were evaluated for their size, zeta potential, and encapsulation efficiency, similar to the characterization of GA-NPs.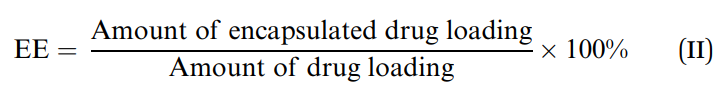
Figure 2. The encapsulation efficiency (EE) was calculated using eqn (II)
Result:
RA-GA-NPs showed a mean particle size of 73.55 ± 0.726 nm and a zeta potential of +41.5 ± 0.78 mV, indicating successful modification with retinoic acid.
Novel Aspect:
This study highlights the use of retinoic acid for targeted delivery to hepatic stellate cells, which enhances the specificity of the drug delivery system compared to conventional nanoparticle systems that lack specific targeting capabilities.
3. In Vitro Drug Release Studies
Primary Technique:
A dialysis method was employed to evaluate the release profile of GA from the nanoparticles.
Key Steps:
- Step 1: Place 1 mL of GA-NPs or RA-GA-NPs into a dialysis bag with a molecular weight cutoff of 8000–12000.
- Step 2: Immerse the dialysis bag in 100 mL of dissolution medium (pH 7.4 phosphate buffer with 0.5% Tween-80) and maintain stirring at 120 rpm at 37 ± 1 °C.
- Step 3: Collect 1 mL of samples at predetermined time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, 72, and 96 hours) and replace with fresh medium.
Data Collection and Analysis:
The released drug concentrations were measured using HPLC, and the cumulative release data was analyzed using the Higuchi equation to determine the release kinetics.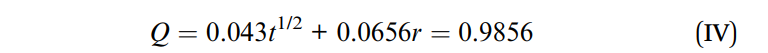
Figure 3. The cumulative release of the RA-GA-NPs curves was consistent with the Higuchi equation and expressed by eqn (IV):
Result:
The release profile indicated an initial burst release of approximately 20% within the first 2 hours, followed by sustained release, with nearly 80% of the total drug released by 96 hours.
Novel Aspect:
The controlled release behavior of the RA-GA-NPs offers a significant advantage in maintaining therapeutic drug levels over an extended period, which is crucial for chronic conditions like hepatic fibrosis.
4. Pharmacokinetic Studies
Primary Technique:
A pharmacokinetic study was performed using Sprague-Dawley rats to evaluate the absorption and distribution of GA from the different formulations.
Key Steps:
- Step 1: Randomly assign 18 rats into three groups: free GA, GA-NPs, and RA-GA-NPs, each receiving a dose of 50 mg/kg of GA orally.
- Step 2: Collect 200 µL blood samples at specific time points (0.5, 1, 2, 4, 6, 8, 10, 12, and 24 hours) post-administration.
- Step 3: Analyze plasma samples using HPLC to determine GA concentrations.
Data Collection and Analysis:
Pharmacokinetic parameters, including peak plasma concentration (Cmax), elimination half-life (t1/2), and area under the concentration-time curve (AUC), were calculated using non-compartmental analysis.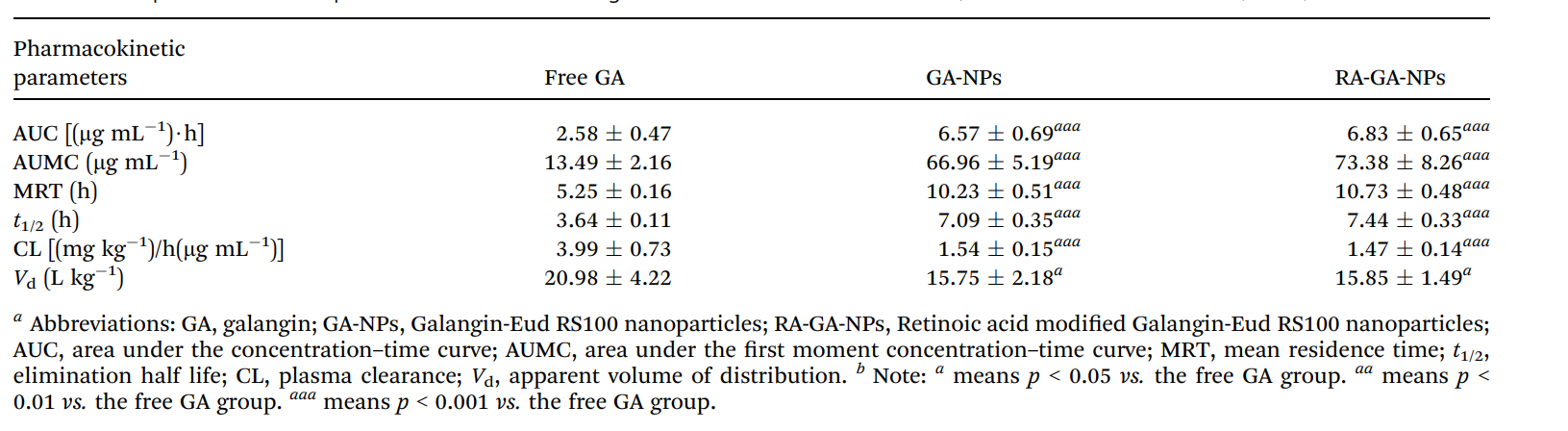
Table 1. The pharmacokinetic parameters of GA following oral administration with free GA, GA-NPs and RA-GA-NPs (n = 6) a,b.
Result:
Both GA-NPs and RA-GA-NPs demonstrated significantly higher serum concentrations and a 2.65-fold increase in AUC compared to free GA, with extended half-lives of 7.09 and 7.44 hours, respectively.
Novel Aspect:
The study demonstrates that nanoparticle formulations markedly enhance the bioavailability of GA, overcoming the limitations of rapid clearance associated with conventional dosage forms.
5. In Vivo Imaging Studies
Primary Technique:
Small animal imaging was utilized to visualize the biodistribution of indocyanine green-loaded nanoparticles in C57BL/6 mice.
Key Steps:
- Step 1: Administer indocyanine green-loaded GA-NPs and RA-GA-NPs (1 mg/kg) orally to groups of mice.
- Step 2: Anesthetize the mice at various time points (0.5, 1, 2, 3, and 4 hours post-administration) and capture fluorescence images using a cryostat microtome.
Data Collection and Analysis:
The fluorescence intensity in the liver was measured and compared across groups to assess the targeting efficiency of the formulations.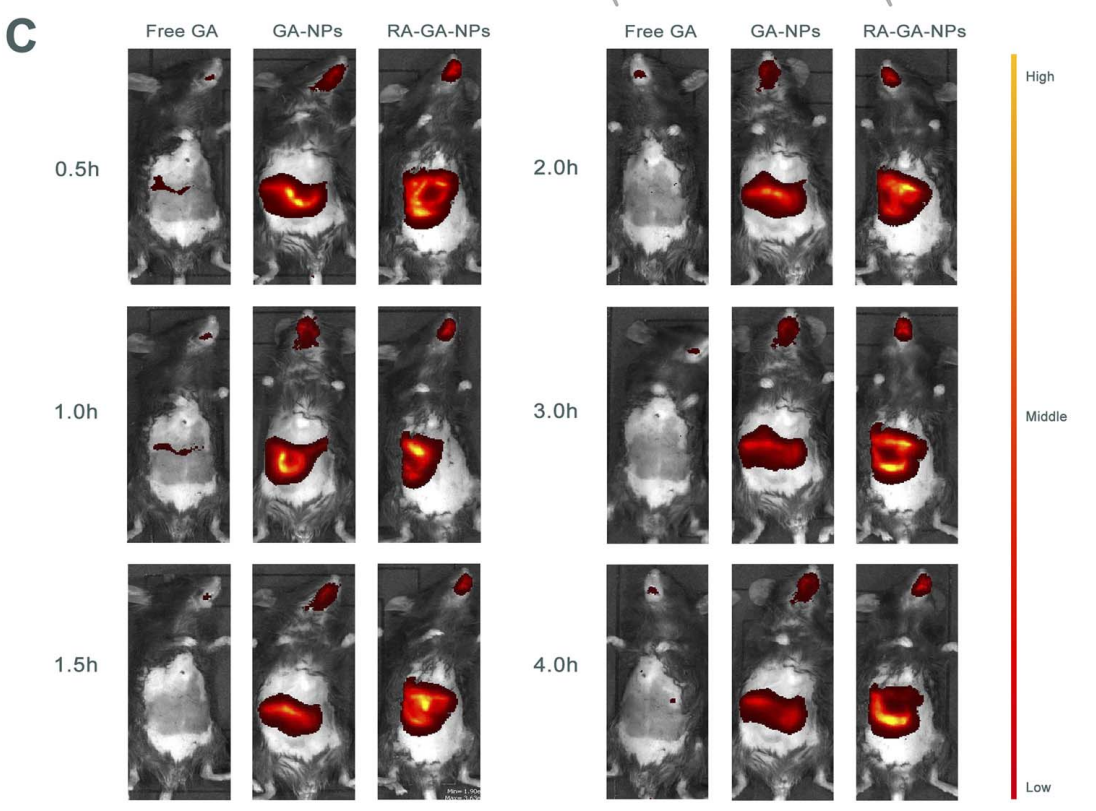
Figure 4. © the representative images of the distribution of GA in mice after single oral administration of free GA, GA-NPs and RA-GA-NPs in 4 h (n = 6).
Result:
The RA-GA-NPs exhibited a significantly higher fluorescence intensity in the liver compared to free GA and GA-NPs, indicating enhanced targeting capabilities.
Novel Aspect:
This imaging technique allows for real-time tracking of nanoparticle distribution, providing insights into the targeting efficiency and potential therapeutic benefit of RA-GA-NPs over traditional delivery systems.
Conclusion
The successful development of the retinoic acid-modified galangin nanoparticles (RA-GA-NPs) as a targeted drug delivery system for the treatment of hepatic fibrosis was achieved through a series of strategic innovations in formulation and targeting techniques. By utilizing a nanoprecipitation method combined with surface modification using retinoic acid, the research team was able to enhance the bioavailability and specificity of galangin, a flavonoid known for its anti-fibrotic properties. The nanoparticles demonstrated favorable physicochemical characteristics, including optimal particle size, high encapsulation efficiency, and a controlled release profile, which collectively contributed to their efficacy.
The highlights of the study include the significant increase in the anti-fibrotic effects of galangin when delivered through RA-GA-NPs, as evidenced by enhanced pharmacokinetic parameters and improved targeting of hepatic stellate cells. The findings also showcased a marked improvement in the therapeutic outcomes for hepatic fibrosis, addressing the limitations of conventional drug delivery methods. Overall, this research represents a promising advancement in the development of effective therapies for hepatic fibrosis, offering a new avenue for targeted treatment strategies in clinical settings.
Reference
Xiong, Yuanguo, et al. “Galangin Delivered by Retinoic Acid-Modified Nanoparticles Targeted Hepatic Stellate Cells for the Treatment of Hepatic Fibrosis.” RSC Advances, vol. 13, 2023, pp. 10987-11001. DOI: 10.1039/d2ra07561j.
