Editor: Nina
Scientists develop a stable and reactive polyethylenimine-CO2 adduct templated calcium carbonate nanoparticle system for effective doxorubicin delivery, enhancing therapeutic efficacy and reducing systemic toxicity in cancer treatment.
Key Preview
- Research Question: The study investigates how to enhance the delivery of anticancer drugs to tumors using calcium carbonate (CaCO3) nanoparticles while maintaining stability and reactivity.
- Research Design and Strategy: Researchers utilized polyethylenimine-CO2 adducts as a template for creating stable, reactive CaCO3 nanoparticles that can effectively deliver the anticancer drug doxorubicin (DOX).
- Method: A combination of chemical synthesis, drug loading, and biological testing was employed to develop the nanoparticles and assess their efficacy in tumor suppression.
- Key Results: The resulting nanoparticles demonstrated long-term stability, effective drug loading, and enhanced tumor targeting, leading to significant tumor growth suppression in mouse models.
- Significance of the Research: This work presents a novel approach to drug delivery that could improve the efficacy of cancer therapies while minimizing side effects.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by the uncontrolled growth and spread of abnormal cells. The complexity of the disease, coupled with the heterogeneity of tumor types and their microenvironments, poses significant challenges in effective treatment. Conventional therapies, including chemotherapy, radiation, and surgery, often aim to eliminate cancer cells; however, they frequently result in adverse effects on healthy tissues, leading to systemic toxicity and reduced patient quality of life.
Traditionally, the delivery of anticancer drugs has relied on systemic administration, where drugs are administered intravenously or orally. This approach allows for a broad distribution of the drug throughout the body, but it also leads to significant challenges, such as poor targeting of tumor cells, inadequate drug concentrations at the tumor site, and the rapid clearance of drugs from circulation. These issues can result in suboptimal therapeutic outcomes, with cancer cells often developing resistance to the treatment, and patients experiencing debilitating side effects.
The current approach to drug delivery in cancer treatment faces distinct challenges, including the difficulty of achieving selective targeting of tumors while minimizing exposure to healthy tissues. Moreover, many anticancer drugs exhibit poor solubility and stability, which complicates their effective administration and bioavailability. As a result, there is a pressing need for innovative strategies that can enhance drug delivery, improve therapeutic efficacy, and reduce side effects.
To address these challenges, recent advancements in nanotechnology have led to the development of novel drug delivery systems, particularly using nanoparticles as carriers. These systems can encapsulate therapeutic agents, providing a means to achieve targeted delivery to tumor cells while sparing healthy tissues. One promising strategy involves using calcium carbonate (CaCO3) nanoparticles, specifically in their vaterite form, which are biodegradable and can dissolve in the acidic microenvironment of tumors. This unique property allows for controlled release of the encapsulated drugs, enhancing efficacy and minimizing toxicity. The current study investigates a novel approach using polyethylenimine-CO2 adduct templated CaCO3 nanoparticles as a carrier for the anticancer drug doxorubicin, aiming to improve therapeutic outcomes in cancer treatment while addressing the limitations of traditional drug delivery methods.
Research Team and Aim
The research was led by Wenli Luo, a prominent investigator from Sichuan University, where he collaborated with co-authors Zhaojian Li, Ling Zhang, and Xingyi Xie. This study was conducted over a period culminating in its publication in January 2023. The paper is titled “Polyethylenimine-CO2 adduct templated CaCO3 nanoparticles as anticancer drug carrier,” and it was published in the journal Cancer Nanotechnology.
The aim of the research, as articulated by lead researcher Wenli Luo, was to “develop a simple, cost-effective method to create a stable and reactive nanoparticle carrier for the delivery of anticancer drugs, particularly doxorubicin, to enhance therapeutic efficacy while reducing systemic toxicity.” This innovative approach seeks to address the longstanding challenges associated with conventional drug delivery systems in cancer treatment.
Experimental Process
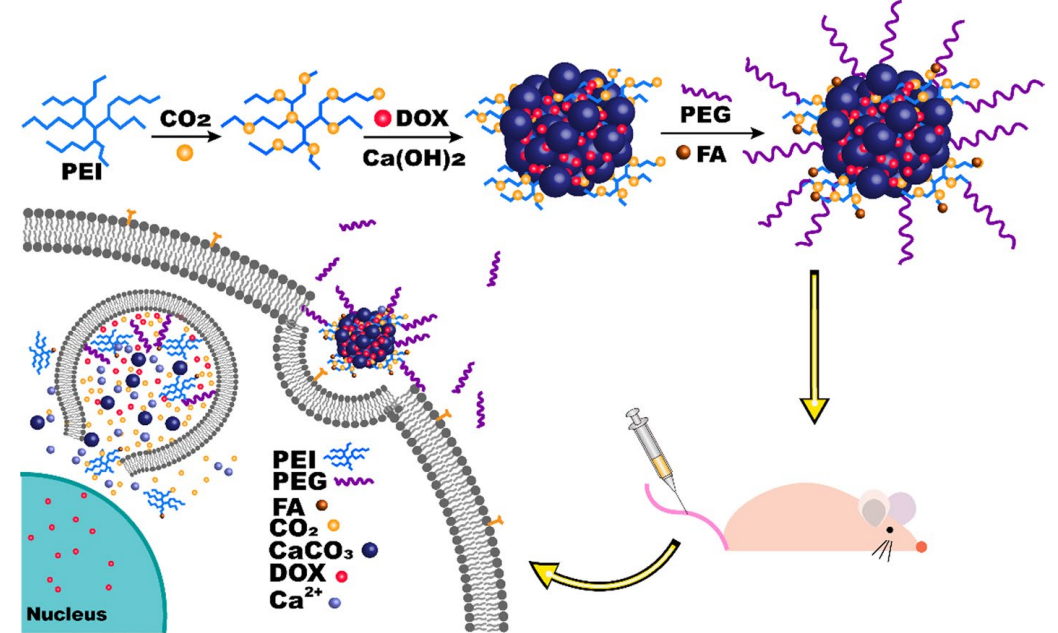
Figure 1. Schematic design of tumor-triggered intracellular drug release of DOX/PEI-CO2@CaCO3-PEG-FA
Experiment 1: Synthesis of CaCO3 Nanoparticles
Primary Technique: The main method employed for synthesizing calcium carbonate (CaCO3) nanoparticles was the templating process using polyethylenimine (PEI)-CO2 adducts as a CO2 source and template for mineralization.
Key Steps:
- Introduce a CO2 atmosphere into 80 mL of a 0.1 g/mL PEI aqueous solution for 48 hours to form the PEI-CO2 adduct.
- Slowly add 800 mL of saturated Ca(OH)2 solution to the PEI-CO2 solution under vigorous stirring at 37 °C for 6 hours.
- Divide the resultant white emulsion into four equal parts for further functionalization.
Data Collection and Analysis:
- Characterization of the synthesized nanoparticles was performed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to assess morphology.
- The crystal structure was analyzed through X-ray diffraction (XRD), while thermogravimetric analysis (TGA) provided insights into thermal stability.
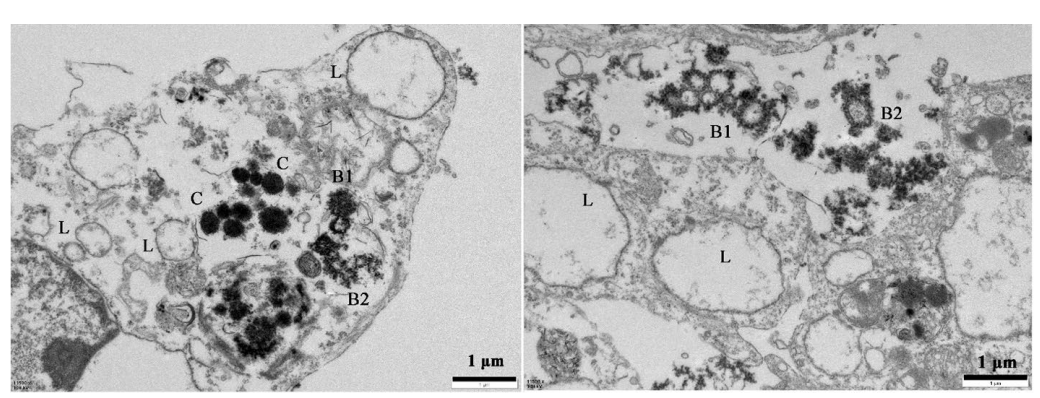
Figure 2. PEI-CO2@CaCO3-PEG-FA particles were entrapped in lysosomes imaged by TEM. L: normal lysosomes; C: particles in lysosomes; B1: bursting lysosomes; B2: burst lysosomes
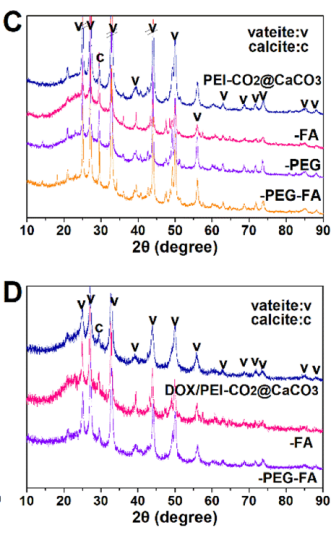
Figure 3. Structural analyses of bare (top) and drug-loaded (bottom) CaCO3 nanoparticles
Result:
- The synthesized nanoparticles exhibited a hydrodynamic diameter of 200-300 nm, with a stable vaterite phase maintained in an aqueous solution for over 8 months.
Novel Aspect:
- This synthesis method demonstrates a simple and economical approach to creating stable and reactive CaCO3 nanoparticles, addressing the stability issues commonly faced in traditional formulations.
Experiment 2: Drug Loading of Doxorubicin (DOX)
Primary Technique: The drug loading of doxorubicin into the CaCO3 nanoparticles was achieved using a coprecipitation method.
Key Steps:
- Prepare a 30 mL solution of DOX at a concentration of 5 mg/mL and add it to 60 mL of PEI-CO2 solution (containing 6 g PEI) under vigorous stirring.
- Gradually add 600 mL of saturated Ca(OH)2 solution over a period of 50 minutes.
- Allow the mixture to react until a translucent purple emulsion is formed and then ultrasonicate for 1-2 minutes to ensure uniform mixing.
Data Collection and Analysis:
- The loading efficiency of DOX in the nanoparticles was determined by analyzing the supernatant using a fluorescence measurement at 588 nm (excitation at 480 nm).
- The drug loading percentage was calculated based on the mass of encapsulated DOX relative to the total mass of the nanoparticles.
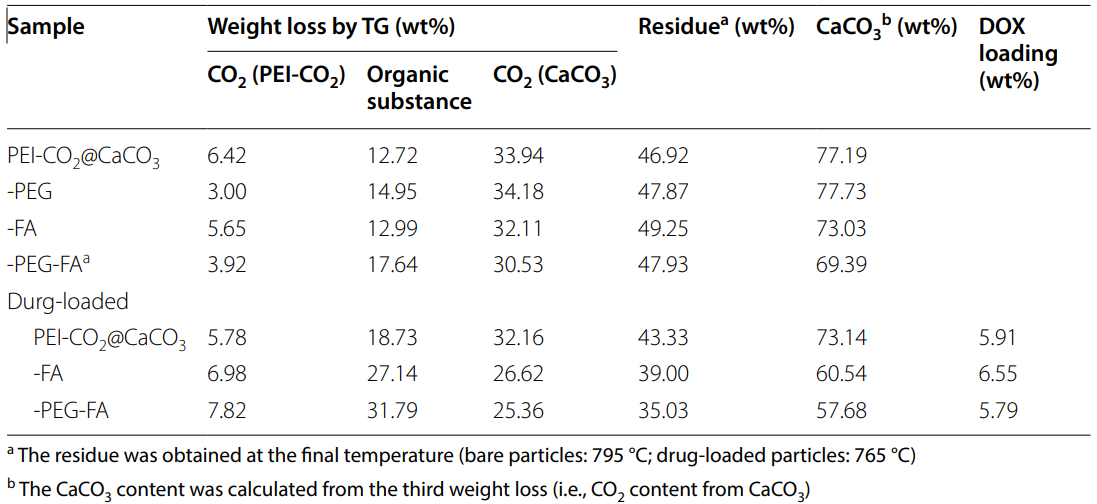
Table 1. Composition of as-synthesized CaCO3 nanoparticles
Result:
- The drug loading efficiency was found to be approximately 5.79-6.55%, indicating effective encapsulation of DOX within the nanoparticles.
Novel Aspect:
- This method of drug loading using CaCO3 nanoparticles not only allows for high loading efficiency but also facilitates a pH-sensitive release mechanism, enhancing therapeutic effectiveness compared to traditional delivery systems.
Experiment 3: In Vitro Drug Release Studies
Primary Technique: The in vitro release profiles of DOX from the drug-loaded nanoparticles were assessed using dialysis membrane techniques under varying pH conditions.
Key Steps:
- Place DOX-loaded nanoparticles into a dialysis bag (MWCO: 5000 Da) and immerse it in 100 mL of phosphate-buffered saline (PBS) at different pH levels (7.4, 6.5, and 5.5).
- Withdraw 5 mL of the releasing medium at predetermined time intervals and replace it with an equal volume of fresh PBS.
- Measure the concentration of released DOX using fluorescence spectroscopy.
Data Collection and Analysis:
- The cumulative release percentages were calculated over time, and the data were analyzed to determine the release kinetics, fitting them to various release models.
Result:
- The release profiles indicated accelerated DOX release at lower pH values, demonstrating the pH-responsive nature of the nanoparticles.
Novel Aspect:
- The study highlights a significant advancement in controlled drug delivery systems by utilizing CaCO3 nanoparticles that respond to the acidic microenvironment of tumors, ensuring targeted and efficient drug release.
Experiment 4: Cytotoxicity Assessment
Primary Technique: Cytotoxicity assays were performed using the Cell Counting Kit-8 (CCK-8) method to evaluate the viability of HeLa cells after exposure to the drug-loaded nanoparticles.
Key Steps:
- Seed HeLa cells in 96-well plates at a density of 5 × 10^3 cells per well and allow them to adhere overnight.
- Replace the medium with serum-free DMEM containing varying concentrations of DOX or CaCO3 nanoparticles.
- Incubate for 24 and 48 hours, followed by the addition of CCK-8 solution and further incubation for 30 minutes.
Data Collection and Analysis:
- Measure the absorbance at 450 nm using a microplate reader to assess cell viability relative to control groups.
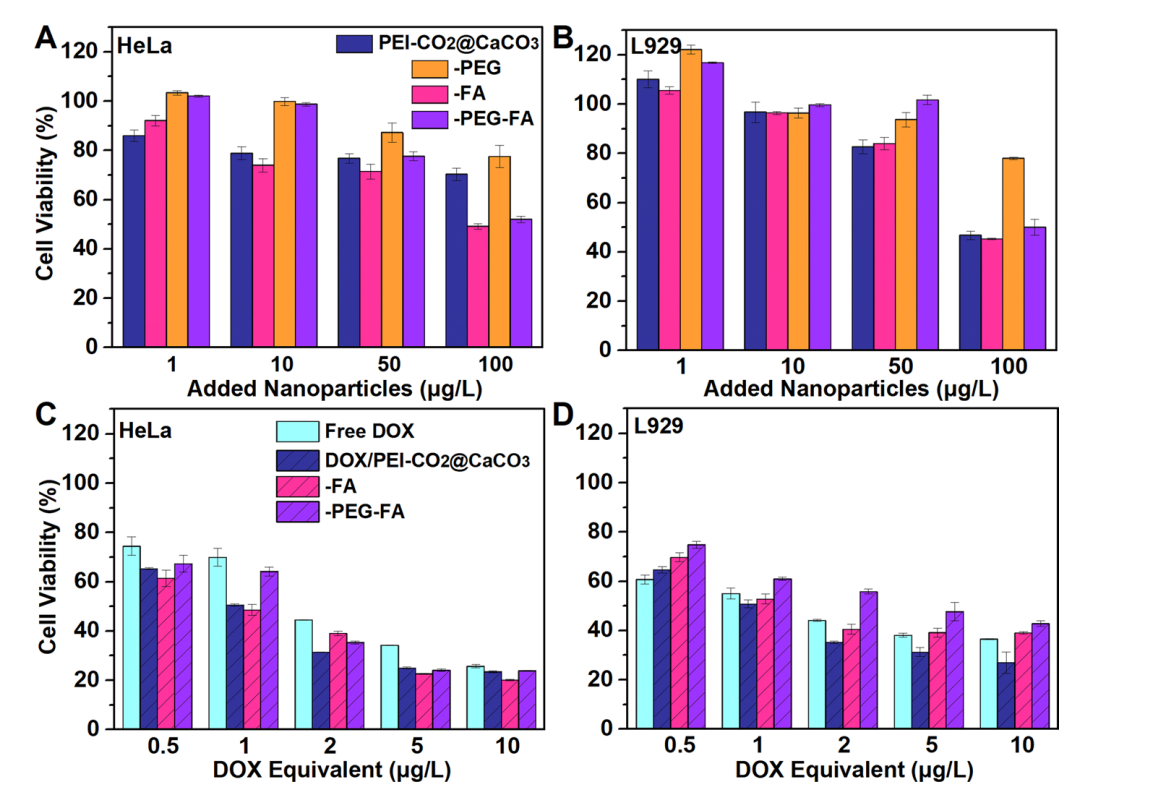
Figure 4. Test of cytotoxicity of as-synthesized CaCO3 nanoparticles. Cell viability is normalized to that of the control group in the DMEM medium
Result:
- The drug-loaded nanoparticles exhibited significant cytotoxic effects at low dosages, indicating their potential for effective cancer treatment.
Novel Aspect:
- This study introduces a novel nanocarrier system that exhibits enhanced cytotoxicity compared to free DOX, thereby improving treatment outcomes in cancer therapy.
Experiment 5: In Vivo Antitumor Efficacy
Primary Technique: The antitumor efficacy of the drug-loaded nanoparticles was evaluated in a HeLa tumor xenograft mouse model.
Key Steps:
- Establish subcutaneous tumors in BALB/c-Nude mice by inoculating 1 × 10^8 HeLa cells.
- Randomly divide the mice into groups and administer 200 μL of saline, free DOX, or DOX-loaded nanoparticles (1 mg/kg DOX equivalent) via tail vein injection every 2 days for 14 days.
- Monitor tumor volumes and mouse body weights throughout the treatment period.
Data Collection and Analysis:
- Tumor volumes were measured using calipers, and the data were analyzed for statistical significance using one-way ANOVA.
Result:
- The DOX-loaded nanoparticles demonstrated significant tumor growth suppression, with the highest efficacy observed in the groups treated with FA and PEG modified formulations.
Novel Aspect:
- The study presents a new method for delivering chemotherapeutics that not only enhances drug efficacy but also reduces systemic toxicity, providing a safer and more effective treatment option for cancer patients.
These experiments collectively illustrate a comprehensive approach to developing and evaluating a novel nanocarrier system for targeted drug delivery in cancer therapy, addressing the limitations of traditional methods while enhancing therapeutic outcomes.
Conclusion
The successful development of the polyethylenimine-CO2 adduct templated CaCO3 nanoparticles as a drug delivery system was achieved through a comprehensive approach that combined innovative synthesis techniques with careful functionalization. By utilizing the PEI-CO2 adduct as both a CO2 source and a template for mineralization, researchers were able to create stable and reactive vaterite nanoparticles that effectively encapsulate the anticancer drug doxorubicin. The incorporation of polyethylene glycol (PEG) and folic acid (FA) further enhanced the nanoparticles’ stability in circulation and facilitated targeted delivery to tumor cells.
The highlights of the study include the demonstration of long-term colloidal stability of the nanoparticles for over eight months, effective drug loading efficiency of 5.79-6.55%, and the ability to achieve accelerated drug release in the acidic tumor microenvironment. The study also showcased significant in vivo antitumor efficacy, underscoring the potential of this novel drug delivery system to improve therapeutic outcomes while minimizing systemic toxicity. Overall, this research represents a significant advancement in the field of cancer nanotechnology, paving the way for more effective and safer cancer therapies.
Reference
Luo, Wenli, Zhaojian Li, Ling Zhang, and Xingyi Xie. “Polyethylenimine-CO2 Adduct Templated CaCO3 Nanoparticles as Anticancer Drug Carrier.” Cancer Nanotechnology, vol. 14, no. 7, Jan. 2023, https://doi.org/10.1186/s12645-023-00156-z.
