Editor: Tiffany
Researchers have developed a fully bioresorbable neural implant that enables simultaneous electrophysiological recording and optogenetic stimulation, offering a safer and more biocompatible solution for neural modulation and monitoring.
Key Highlights
- Research Question:
How can the challenges of traditional neural implants, such as the need for secondary surgeries and mechanical mismatches with brain tissue, be addressed through a fully bioresorbable neural implant capable of both recording and stimulation? - Research Difficulties:
Designing a multifunctional bioresorbable device that integrates diverse materials and functions without causing unwanted interactions or mechanical failures, while ensuring biocompatibility and functionality in vivo. - Key Findings:
The study demonstrated successful in vivo performance with minimal photoelectric artifacts (less than 0.1 mV), high biocompatibility with no significant tissue damage or inflammation, and complete dissolution of the device within 60 days. - Innovative Aspects:
The use of biodegradable materials such as poly(lactic-co-glycolic acid) (PLGA) and molybdenum/silicon (Mo/Si) for a multifunctional neural interface that combines optical stimulation and electrical recording. - Importance of the Study:
This research represents a significant step forward in neural interface technology, offering a safer and more biocompatible option for treating neurological disorders by eliminating the need for secondary surgeries and reducing tissue damage.
Challenges in Neural Implant Technology and the Need for Bioresorbable Solutions
Neural implants are devices that interface directly with the nervous system, providing a means to identify brain regions functionally and offering potential clinical treatments for neurological disorders such as Parkinson’s disease, Alzheimer’s disease, and epilepsy. The paper states that these implants “have provided a route to functional identification of brain regions and a promising approach to clinical treatment” for such conditions. However, traditional micro-neural implant electronics (μ-NIE), typically constructed from silicon or metals, have a high Young’s modulus (over 100 GPa), which contrasts with the softness of brain tissue (3 kPa). This mismatch results in “acute tissue damage and inflammatory responses,” leading to glial scarring and neuronal loss.
Even flexible and soft neural implants, while improving biocompatibility, require secondary surgeries for removal, introducing risks of additional tissue damage and complications. The paper notes that “secondary surgeries for device removal” remain a significant limitation. To address these issues, researchers developed a fully bioresorbable hybrid opto-electronic neural implant system. This device, made from biodegradable materials, dissolves naturally in the body after its functional period, eliminating the need for removal surgeries. Its flexible and soft design ensures “conformal contact with the curved surface of the cerebral cortex,” improving biocompatibility and reducing mechanical mismatches with brain tissue.
Developing a Bioresorbable Neural Implant for Simultaneous Recording and Stimulation
The study aimed to develop a flexible, biocompatible neural implant using biodegradable materials that integrates optical and electrical interfaces for neural modulation and monitoring. The paper highlights the challenge of “designing multifunctional bioresorbable devices,” noting difficulties in integrating diverse materials and functions without causing “unwanted interactions or mechanical failures.” The objectives were: (1) to fabricate the device using biodegradable materials such as poly(lactic-co-glycolic acid) (PLGA) and molybdenum/silicon (Mo/Si), (2) to ensure its biocompatibility with brain tissue, and (3) to demonstrate its functionality in vivo through simultaneous electrophysiological recording and optogenetic stimulation. The research team, affiliated with Yonsei University and the Korea Institute of Science and Technology, published their findings in Nature Communications on March 6, 2024.
Research Methods & Results
(1) Experimental Process Outline
- Design of a fully bioresorbable flexible hybrid opto-electronic neural implant system.
- Fabrication of the PLGA waveguide and Mo/Si electrode array.
- Integration of the waveguide with the electrode array.
- Characterization of electrical properties using electrochemical impedance spectroscopy (EIS).
- In vitro testing of the device in a biomimetic hydrogel.
- Chronic implantation in Thy-1: Channelrhodopsin-2 (ChR2) transgenic mice.
- Recording of local field potentials (LFPs) during optical stimulation.
- Evaluation of device biocompatibility through cell viability tests.
- Immunohistochemistry analysis for glial activation.
- Monitoring of device degradation over time using computed tomography.
(2) Key Experiments
Experiment 1: In Vitro Characterization of the Device
- Procedure: The device was immersed in phosphate-buffered saline (PBS) at room temperature for electrochemical impedance spectroscopy (EIS) measurements. The impedance values were recorded at varying frequencies (1 Hz to 10 kHz).
- Result: The impedance characteristics showed minimal variation when the device was bent, indicating good mechanical stability and performance.
- Finding: The device maintained stable electrical properties, confirming its suitability for long-term implantation.
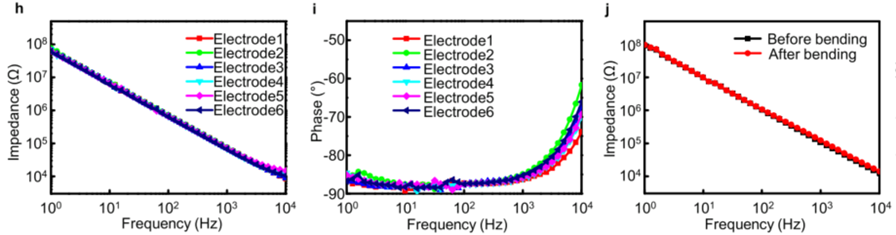
Figure 1. (h) Magnitude, (i) phase, and (j) impedance spectra of the biodegradable electrode array under flat and bent states with a 2 mm bending radius.
Experiment 2: Chronic In Vivo Implantation and Recording
- Procedure: The bioresorbable hybrid device was implanted into the cerebral cortex of Thy-1: ChR2 transgenic mice. Neural activities were recorded while administering blue light stimulation (460 nm) to activate ChR2-expressing neurons.
- Result: The device successfully recorded LFPs for up to 14 days, with evoked potentials demonstrating clear responses to optical stimulation.
- Finding: The hybrid device can perform continuous electrophysiological recordings while providing targeted optogenetic stimulation, validating its dual functionality.
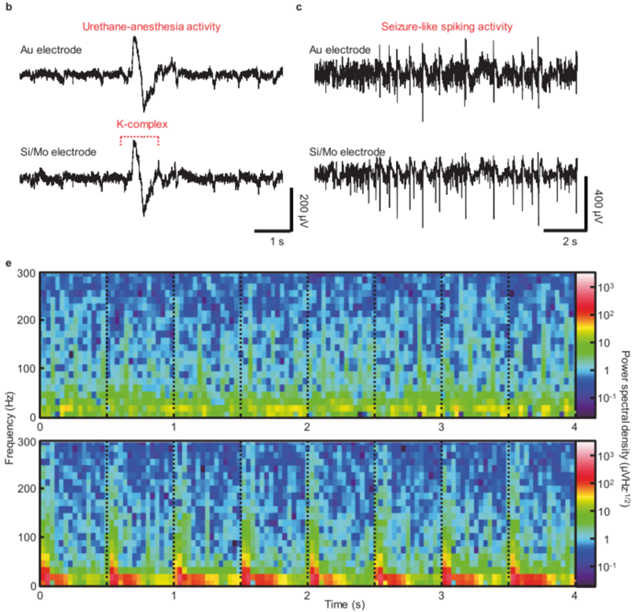
Figure 2. (b)Spontaneous and (c) spiking activities captured by the bioresorbable and control electrodes. (e) Spectrogram of spontaneous activities (top) and evoked LFPs (bottom) power spectral density.
Experiment 3: Biocompatibility and Immune Response Assessment
- Procedure: Primary hippocampal neurons were cultured on the bioresorbable electrode arrays to assess cell viability using live/dead assays. Immunohistochemistry was performed on brain tissue sections post-implantation to evaluate astrocyte and microglial activation.
- Result: High cell survival rates were observed on the bioresorbable electrodes. Immunohistochemistry results showed moderate gliosis with no significant differences in immune response between the implantation site and control areas.
- Finding: The device exhibited high biocompatibility, with a minimal inflammatory response, suggesting its safety for in vivo applications.
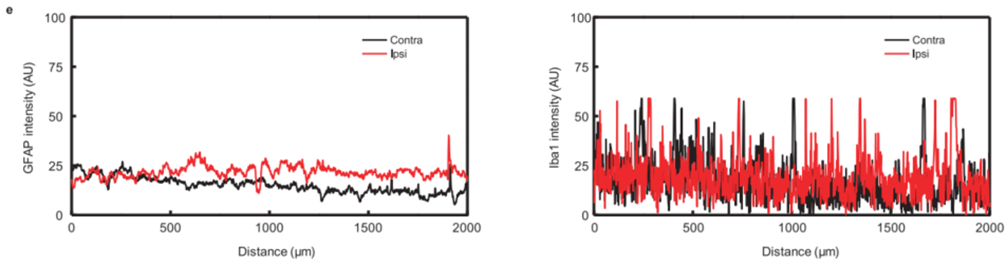
Figure 3. Comparison of immune response by GFAP and Iba-1 intensity (AU = arbitrary units).
Implications of the Bioresorbable Neural Implant for Future Neural Interface Applications
This study developed a fully bioresorbable hybrid opto-electronic neural implant capable of simultaneous electrophysiological recording and optogenetic stimulation. Key findings include: (1) effective in vivo performance with minimal photoelectric artifacts (less than 0.1 mV), (2) high biocompatibility with no significant tissue damage or inflammation, and (3) complete dissolution in PBS within 60 days, eliminating the need for secondary surgeries. The use of biodegradable materials such as PLGA and Mo/Si enabled a multifunctional neural interface that addresses limitations of traditional implants. The paper concludes that this technology “represents a significant step forward in the development of next-generation neural interfaces,” with potential implications for treating neurological disorders by offering a safer and more biocompatible option.
Reference:
Cho, Myeongki, et al. “Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation.” Nature communications 15.1 (2024): 2000.
