Editor: Nina
Scientists develop large pore mesoporous silica nanoparticles as an innovative drug delivery system for effective siRNA targeting of VEGF in retinal cells to enhance treatment for age-related macular degeneration.
Key Preview
Research Question
The study addresses the challenges in treating wet age-related macular degeneration (AMD), particularly the ineffective delivery and retention of anti-VEGF drugs. It investigates whether large pore mesoporous silica nanoparticles (LP-MSNs) can effectively transport and deliver siRNA targeting VEGF to retinal pigment epithelial (RPE) cells, thus providing a more efficient therapeutic strategy.
Research Design and Strategy
The research employs a systematic approach using LP-MSNs as nanocarriers to enhance the delivery of siRNA to RPE cells. This involves synthesizing and functionalizing LP-MSNs for optimal loading and controlled release of the siRNA, followed by in vitro assessments of their efficacy.
Method
The researchers synthesized LP-MSNs and functionalized them with polyethylenimine (PEI) to create a nanosystem capable of loading siRNA. The efficacy of these carriers was tested by assessing VEGF silencing in ARPE-19 cells, a human RPE cell line, after treatment with the siRNA-loaded LP-MSNs.
Key Results
The study found that the LP-MSNs effectively silenced VEGF expression in ARPE-19 cells, achieving a knockdown of up to 75% at 72 hours post-treatment. The nanocarriers demonstrated biocompatibility and efficient cellular uptake, indicating their potential as a viable option for retinal delivery of siRNA.
Significance of the Research
This research is significant as it proposes a novel nanocarrier system for the effective delivery of therapeutic siRNA to the retina, addressing the limitations of current treatment methods for AMD. The findings suggest that LP-MSNs could enhance the stability, bioavailability, and efficacy of ocular gene therapies.
Introduction
Age-related macular degeneration (AMD) is a leading cause of vision loss among older adults, characterized by the degeneration of the macula, the central part of the retina responsible for sharp and detailed vision. AMD manifests in two forms: dry and wet, with the latter being associated with subretinal neovascularization driven by the overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium (RPE). This abnormal growth can lead to significant vision impairment and even blindness, making it imperative to develop effective therapeutic strategies to manage the disease.
Traditional treatment methods for wet AMD primarily involve the intravitreal injection of anti-VEGF monoclonal antibodies and other gene therapy products. While these approaches have proven effective in controlling neovascularization and preserving vision, they come with considerable drawbacks. Frequent administration of injections is required—often monthly or bi-monthly—which can lead to patient non-compliance due to discomfort, anxiety, and the risk of complications such as infection and retinal detachment. Moreover, the physiological barriers of the eye limit the bioavailability of therapeutics administered through topical or systemic routes, often resulting in less than 5% of the drug reaching the target tissues.
These challenges underscore the urgent need for innovative drug delivery systems that can enhance the stability, penetration, and retention of therapeutic agents within the ocular environment. The proposed study introduces a novel strategy utilizing large pore mesoporous silica nanoparticles (LP-MSNs) as nanocarriers for the effective delivery of small interfering RNA (siRNA) targeting VEGF to the RPE cells. This approach aims to overcome the limitations of current treatments by providing a more efficient and sustained release of therapeutic agents, thereby improving patient adherence and therapeutic outcomes in the management of AMD. Through the encapsulation of siRNA within LP-MSNs, the study seeks to revolutionize the treatment landscape for retinal diseases, addressing both efficacy and safety concerns associated with traditional drug delivery methods.
Research Team and Aim
The research team behind this study was a multidisciplinary group of experts in the fields of nanomedicine and ophthalmology. The research was led by Dr. Amelia Ultimo, who conducted this investigation in collaboration with her colleagues Mar Orzaez, Maria J. Santos-Martinez, Ramón Martínez-Máñez, María D. Marcos, Félix Sancenón, and Eduardo Ruiz-Hernández. The research was carried out at Trinity College Dublin and the Universitat Politècnica de València, with findings published in the paper titled “High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery” in the International Journal of Molecular Sciences.
The aim of the research, as articulated by Dr. Ultimo, was to “develop an LP-MSN-based nanosystem for the administration and controlled delivery of anti-VEGF siRNA molecules, and it’s in vitro assessment in retinal pigmented epithelial cells.” This objective underscores the team’s commitment to innovating drug delivery methods that address the critical limitations of current therapies for age-related macular degeneration.
Experimental Process
Experiment 1: Synthesis of Large Pore Mesoporous Silica Nanoparticles (LP-MSNs)
Primary Technique: The primary technique employed for synthesizing LP-MSNs followed a surfactant templating method, which facilitates the creation of large pores suitable for drug loading.
Key Steps:
- Prepare a solution by dissolving 0.96 g of cetyltrimethylammonium p-toluenesulfonate (CTATos) and 0.1735 g of triethanolamine (TEA) in 50 mL of ultrapure water.
- Stir the mixture at 80 °C and 400 rpm for 1 hour.
- Add 7.8 mL of tetraethylorthosilicate (TEOS) rapidly to the stirred solution and continue stirring at 80 °C at 1000 rpm for an additional 2 hours.
- Collect the resulting nanoparticles via centrifugation, wash them three times with ultrapure water, and dry at 60 °C overnight.
- Calcine the dried nanoparticles at 550 °C for 5 hours to remove the surfactant template.
Data Collection and Analysis: The morphology and size of the synthesized LP-MSNs were analyzed using transmission electron microscopy (TEM). Additional characterization included dynamic light scattering (DLS) to determine the hydrodynamic size and surface area measurements through nitrogen adsorption/desorption isotherms.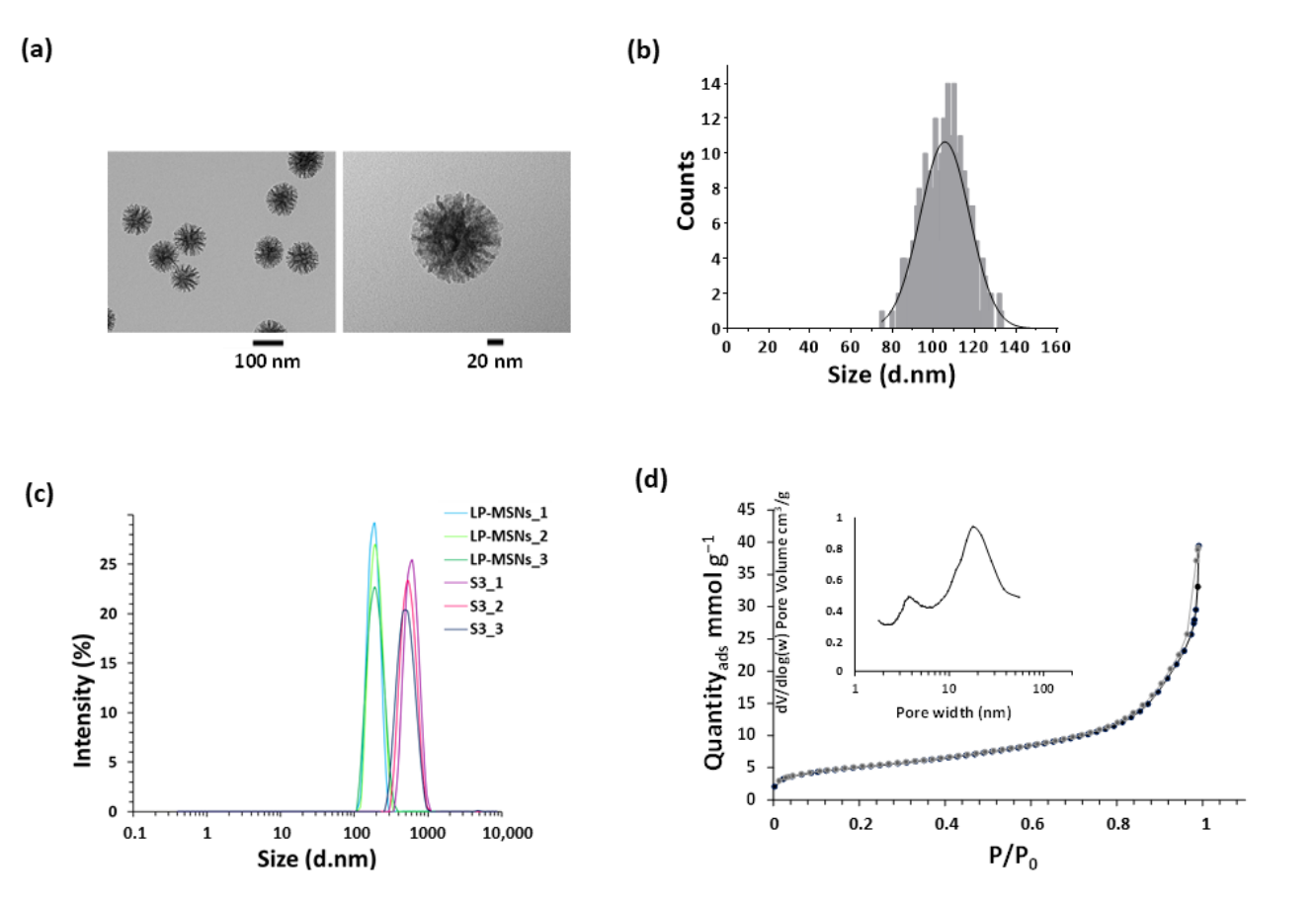
Figure 1. LP-MSN characterisation. (a) Representative transmission electron microscopy (TEM) images of LP-MSNs. (b) LP-MSN size distribution obtained by measurements taken from TEM images. © Dynamic light scattering (DLS) intensity distribution of LP-MSNs and S3 nanoparticles size. Three independent measurements for each type of nanomaterials are represented.
Result: The synthesized LP-MSNs exhibited a spherical morphology with an average size of 105 ± 12 nm and a significant surface area of 398 m²/g, confirming the successful formation of mesoporous structures.
Novel Aspect: The study introduces a scalable synthesis method for LP-MSNs that ensures a high loading capacity and large pore sizes. This advancement provides a more efficient platform for drug delivery compared to traditional silica nanoparticles, which often have smaller pore sizes and lower capacities.
Experiment 2: Functionalization of LP-MSNs with Polyethylenimine (PEI)
Primary Technique: The functionalization of LP-MSNs involved covalently attaching branched polyethylenimine (PEI) to the surface of the mesoporous silica nanoparticles.
Key Steps:
- Suspend 150 mg of the synthesized LP-MSNs in 10 mL of ethanol.
- Add 75 mg of PEI (molecular weight: 10,000) to the suspension and stir for 3 hours to allow for electrostatic interactions between the PEI and the LP-MSN surface.
- Centrifuge the mixture to isolate the functionalized nanoparticles, wash them with ethanol, and dry under vacuum.
Data Collection and Analysis: The zeta potential of the functionalized LP-MSNs was measured using a Zetasizer Nano ZS to confirm successful PEI surface modification. A change in zeta potential indicates successful attachment of the positively charged PEI.
Result: The zeta potential shifted from −40.4 mV for bare LP-MSNs to +27.2 mV post-functionalization, confirming the successful coating of PEI on the nanoparticles.
Novel Aspect: The use of PEI as a capping agent enhances the controlled release of cargo from the LP-MSNs and promotes endosomal escape, which is a significant improvement over traditional delivery systems that lack such functionalities.
Experiment 3: siRNA Loading into LP-MSNs
Primary Technique: The loading of siRNA into LP-MSNs was conducted using a solvent-assisted method to ensure efficient encapsulation within the mesopores.
Key Steps:
- Combine 56 µL of siRNA solution with 0.7 mg of LP-MSNs and add 70 µL of a 4 M guanidine hydrochloride solution and 280 µL of ethanol.
- Vortex the mixture to ensure uniform distribution and incubate it at 25 °C while shaking at 270 rpm for 1 hour.
- Centrifuge to separate the unbound siRNA and measure the amount of siRNA loaded into the nanoparticles.
Data Collection and Analysis: The quantity of loaded siRNA was determined using a NanoDrop Spectrophotometer, measuring nucleic acid absorbance before and after loading to calculate the loading efficiency.
Result: An amount of 45.6 µg of siRNA per mg of LP-MSNs was achieved, corresponding to a loading efficiency of 43%.
Novel Aspect: This method enhances the stability of siRNA by encapsulating it within large pores, providing protection against enzymatic degradation, which is a common issue with traditional siRNA delivery systems.
Experiment 4: Release Profile of siRNA from LP-MSNs
Primary Technique: The release profile of siRNA from the LP-MSNs was evaluated under controlled conditions simulating endosomal environments.
Key Steps:
- Place 2 mg of siRNA-loaded LP-MSNs into a release medium consisting of purified lysosomal extract and incubate at 37 °C.
- Withdraw aliquots at predetermined time points and measure the fluorescence intensity of released siRNA using a spectrophotometer.
Data Collection and Analysis: The release data were quantified by comparing the fluorescence measurements to a standard curve, allowing calculation of the cumulative release percentage over time.
Result: The siRNA-loaded LP-MSNs demonstrated a significant increase in release rate in the lysosomal environment compared to control conditions, indicating effective responsiveness of the nanocarriers.
Novel Aspect: The stimuli-responsive release mechanism showcases a significant advancement over traditional systems, allowing for targeted delivery and controlled release of siRNA in therapeutic applications.
Experiment 5: In Vitro Assessment of VEGF Silencing in ARPE-19 Cells
Primary Technique: The efficacy of the siRNA-loaded LP-MSNs to silence VEGF expression in ARPE-19 retinal pigment epithelial cells was evaluated using an ELISA method.
Key Steps:
- Seed ARPE-19 cells in 6-well plates at a density of 250,000 cells per well and allow them to adhere for 24 hours.
- Treat the cells with varying concentrations of siRNA-loaded LP-MSNs and incubate for 6 hours at 37 °C.
- Replace the media with fresh DMEM/F-12 and collect the supernatants 48 and 72 hours post-treatment for VEGF quantification.
Data Collection and Analysis: The levels of VEGF in the cell supernatants were measured using a Quantikine ELISA Human VEGF kit, with statistical analysis performed to determine the significance of the results.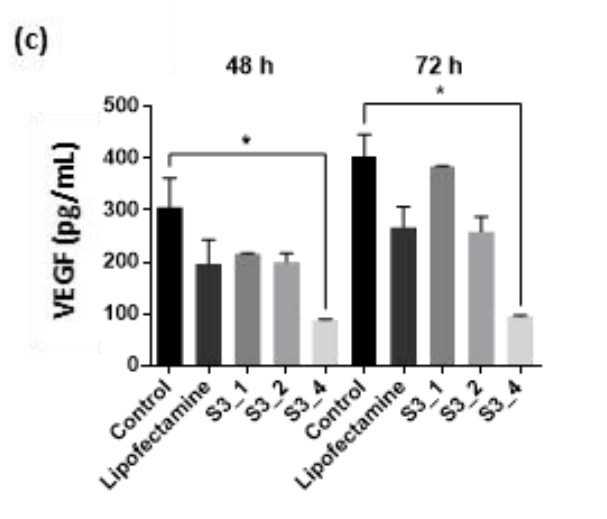
Figure 2. VEGF levels (pg/mL) in ARPE-19 cell supernatant after transfection with 100 nM siRNA/lipofectamine complex or treatment with 1× (S3_1), 2× (S3_2), or 4× (S3_4) the amount of siRNA in lipofectamine complex. Data are expressed as means ± s.d. Asterisks indicate significant differences (p < 0.05) when the t-test was applied.
Result: Treatment with siRNA-loaded LP-MSNs resulted in a VEGF knockdown of up to 75% at 72 hours, demonstrating the efficiency of the delivery system.
Novel Aspect: This experiment illustrates the successful application of the LP-MSNs as a delivery vehicle for siRNA, highlighting their potential to achieve significant gene silencing effects, which is a notable improvement over existing delivery systems that often fail to deliver effective concentrations of therapeutic agents to target cells.
Conclusion
The successful development of the large pore mesoporous silica nanoparticles (LP-MSNs) as a drug delivery system for siRNA targeting VEGF represents a significant advancement in the treatment of age-related macular degeneration (AMD). This innovative approach effectively addresses the challenges associated with traditional therapies, such as frequent intravitreal injections and poor bioavailability of therapeutic agents. By leveraging the unique properties of LP-MSNs, including their high loading capacity, controlled release mechanisms, and ability to enhance cellular uptake, this study demonstrates a promising alternative for delivering therapeutic nucleic acids to retinal cells.
A highlight of the study is the achievement of a VEGF knockdown of up to 75% in ARPE-19 cells following treatment with siRNA-loaded LP-MSNs, underscoring the efficacy of this novel delivery system. Furthermore, the biocompatibility and efficient cellular uptake of the LP-MSNs indicate their potential for safe and effective application in ocular gene therapies. Overall, the findings from this research pave the way for further optimization and in vivo studies, with the ultimate goal of translating this innovative drug delivery strategy into clinical practice for the management of retinal diseases.
Reference
Ultimo, Amelia, et al. “High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery.” International Journal of Molecular Sciences, vol. 24, no. 3, 2023, p. 2753. MDPI, https://doi.org/10.3390/ijms24032753.
