Editor: Sarah
As global antibiotic resistance continues to intensify, antimicrobial peptides (AMPs) have gained attention as potential alternatives to traditional antibiotics. These peptides, naturally present in many organisms, possess broad-spectrum antibacterial properties and operate through membrane disruption rather than specific enzymatic pathways, thereby minimizing the risk of resistance development. However, their clinical potential is constrained by two major drawbacks: rapid degradation by proteases and cytotoxicity to mammalian cells.
This study explores a strategy to overcome these challenges through the use of silica particles as a protective delivery medium. By forming a complex between a cell-penetrating peptide (CPP) and the AMP KR12, and encapsulating this conjugate (CPP-KR12) within silica matrices, the research investigates improvements in peptide stability, efficacy, and biocompatibility for potential use in medical devices and tissue regeneration.
Contribution to the Field
While previous research has attempted to enhance AMP stability through chemical modification and synthetic mimics, few approaches have effectively addressed degradation and toxicity simultaneously. This study introduces an alternative method based on biomimetic silicification, leveraging the natural cationic properties of peptides to entrap them in silica.
The following are the key contributions and findings of the research:
Enhanced Stability Against Proteolysis:
The CPP-KR12@Si complex exhibited substantially improved resistance to protease degradation. After trypsin treatment, CPP-KR12@Si retained approximately 45% of its antibacterial activity, whereas free KR12 showed nearly complete loss of function.
The high entrapment efficiency (95.45%) and loading efficiency (76.31%) of CPP-KR12 in silica particles support the system’s suitability for protecting AMPs during storage and in biological environments.
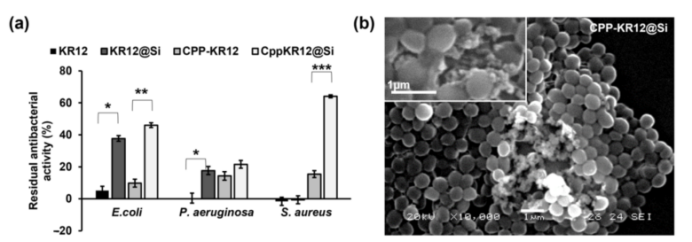
Figure 1: Residual antibacterial activity after protease treatment and SEM image of S. aureus.
Improved Antimicrobial Activity:
The CPP-KR12 conjugate demonstrated significantly lower minimum inhibitory concentration (MIC) values compared to KR12 alone: 12.09 μM vs. 103.36 μM for E. coli, and 6.12 μM vs. 181.98 μM for P. aeruginosa. KR12 alone did not show activity against S. aureus at the tested concentrations, while CPP-KR12 achieved a MIC of 22.80 μM.
CPP-KR12@Si particles exhibited strong bactericidal effects, as seen in electron microscopy images showing bacterial membrane disruption and aggregation upon exposure.
Reduced Cytotoxicity and Hemolytic Activity:
In cytotoxicity assays using RAW 264.7 macrophages, CPP-KR12@Si showed lower toxicity than its free counterpart, with increased cell viability and reduced hemolytic effects. Hemolytic activity remained below 1% at concentrations under 4.84 μM.
Free silica particles had a greater hemolytic effect than AMP-loaded particles, indicating that peptide entrapment can mitigate the inherent risks of nanoparticle use.
Potential Anti-Inflammatory Effect:
The peptide complex demonstrated the ability to reduce lipopolysaccharide (LPS)-induced inflammatory responses in vitro. CPP-KR12 showed enhanced inhibition of IL-6 expression, while both CPP-KR12 and KR12 decreased levels of TNF-α and IL-1β in LPS-stimulated macrophages.
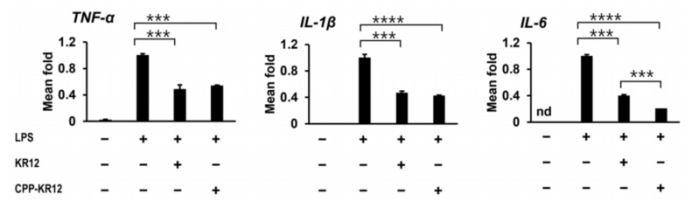
Figure 2: Anti-inflammatory response of AMPs in LPS-stimulated RAW264.7 cells.
Feasibility of Medical Device Integration:
The study demonstrated successful coating of β-tricalcium phosphate (β-TCP) bone graft substitutes (BGS) with CPP-KR12@Si particles. These coatings exhibited effective antibacterial activity against E. coli, suggesting applicability in surgical implants and orthopedic devices.
Scanning electron microscopy confirmed uniform silica deposition on BGS surfaces, and X-ray photoelectron spectroscopy validated the chemical composition of the coating.
Mechanistic Insights into Peptide Functionality:
Gel retardation assays confirmed CPP-KR12’s DNA-binding capacity, which may contribute to intracellular antimicrobial effects. CPP facilitated enhanced uptake of KR12 by increasing membrane permeability without disrupting mammalian cells, indicating its utility as a delivery enhancer.
The study also referenced reactive oxygen species (ROS) generation as a potential bactericidal mechanism associated with LL-37-derived peptides like KR12.
Methodology
The peptides KR12 and CPP were synthesized and conjugated via a GSS linker to form CPP-KR12. Biomimetic silicification was performed in the presence of phosphate ions to produce CPP-KR12@Si particles. Loading and entrapment efficiencies were assessed via quantification of peptide content post-silicification. MIC values were determined against E. coli, P. aeruginosa, and S. aureus. Cytotoxicity was tested using live/dead cell assays, and hemolytic activity was evaluated using sheep red blood cells. Surface morphology and elemental composition were examined via SEM and XPS.
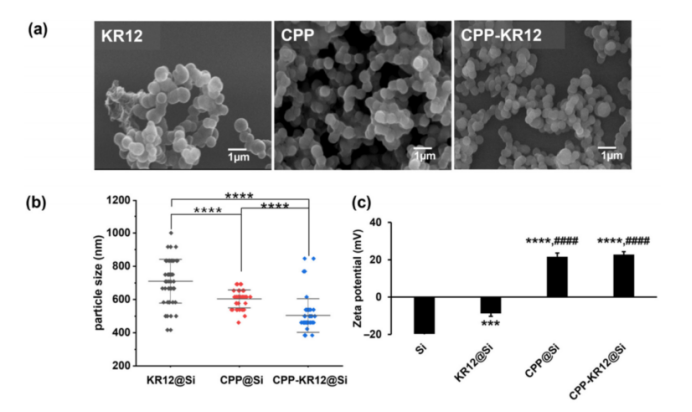
Figure 3: Shape and properties of silica particles formed by each AMP.
Implications and Applications
This research contributes to the ongoing development of localized antimicrobial delivery systems. The silica entrapment approach supports the controlled release of AMPs at infection-prone sites, potentially reducing systemic antibiotic use. It is especially relevant for coatings on implants, where stable and sustained antimicrobial protection is needed. Furthermore, the immunomodulatory potential of CPP-KR12@Si enhances its appeal for tissue engineering applications where both infection control and inflammation management are critical.
The ability to co-opt natural silica-depositing mechanisms for peptide delivery introduces a novel direction for combining therapeutic efficacy with biocompatibility, offering new possibilities for infection-resistant materials in clinical settings.
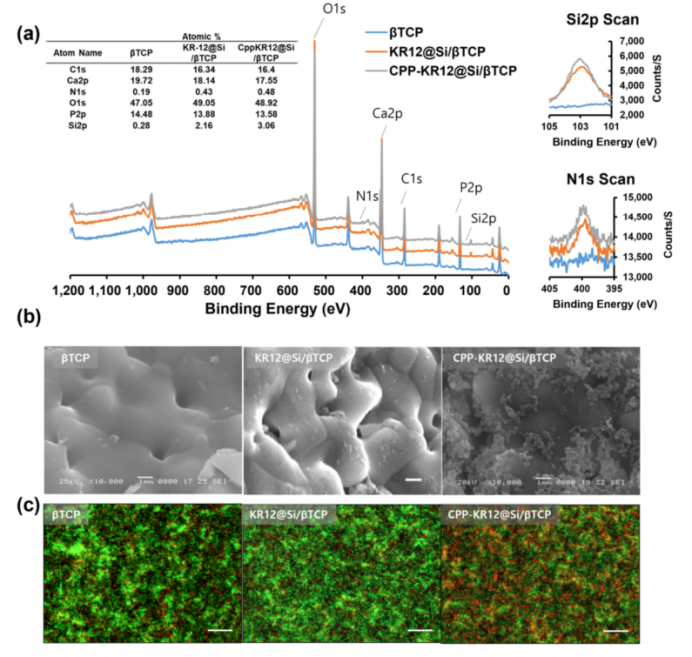
Figure 4: AMP-device combination and antimicrobial activity.
Conclusion
This study provides a practical approach for improving the clinical viability of antimicrobial peptides through biomimetic silica entrapment. By enhancing proteolytic stability, minimizing toxicity, and maintaining strong antibacterial efficacy, the CPP-KR12@Si system demonstrates potential for use in drug-device combination products. Its adaptability for coating medical implants and promoting tissue regeneration offers promising implications for combating antibiotic resistance and improving patient outcomes in infection-prone clinical scenarios.
Reference
Ki, Mi-Ran, et al. “Self-Entrapment of Antimicrobial Peptides in Silica Particles for Stable and Effective Antimicrobial Peptide Delivery System.” International Journal of Molecular Sciences, vol. 24, no. 22, 2023, article 16423, https://doi.org/10.3390/ijms242216423. Accessed 30 Apr. 2025.
