Editor: Nina
Scientists develop a mitochondria-targeting nanoplatform, MiBaMc, synthesized from Prussian blue nanoparticles using Shewanella oneidensis MR-1, to enhance checkpoint blockade immunotherapy and achieve significant tumor growth inhibition in triple-negative breast cancer and colorectal cancer models.
Key Preview
- Research Question: This study investigates how to enhance the efficacy of checkpoint blockade immunotherapy in cancer treatment by utilizing microbial synthesis of Prussian blue nanoparticles.
- Research Design and Strategy: The research employs a biological precipitation method using electrochemically active bacteria, specifically Shewanella oneidensis MR-1, to create FDA-approved Prussian blue nanoparticles and combine them with phototherapy and immune checkpoint inhibitors.
- Method: The method involves the cultivation of S. oneidensis in a controlled environment to produce Prussian blue nanoparticles, which are then modified to create a mitochondria-targeting nanoplatform named MiBaMc.
- Key Results: The study reports a tumor growth inhibition of 95.5% in a triple-negative breast cancer model and 89.4% in a colorectal cancer model when MiBaMc is combined with anti-PDL1 antibodies and phototherapy.
- Significance of the Research: This research demonstrates a novel approach to enhancing cancer immunotherapy by integrating nanotechnology and microbial synthesis, which may improve patient responses and overcome limitations of current therapies.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by the uncontrolled growth and spread of abnormal cells. Among the various forms of cancer, triple-negative breast cancer (TNBC) and colorectal cancer are particularly aggressive and notoriously difficult to treat due to their heterogeneous nature and lack of targeted therapies. Traditional treatment modalities, such as chemotherapy and radiation, have been the mainstays in combating these malignancies; however, these approaches often result in limited efficacy and significant toxicity to healthy tissues.
A common strategy for drug delivery in conventional cancer therapies involves the systemic administration of chemotherapeutic agents. This method aims to ensure that the drugs reach the tumor site effectively; however, it is typically accompanied by challenges such as poor bioavailability, rapid clearance from the bloodstream, and the inability of therapeutic agents to selectively target cancer cells. As a result, many patients experience severe side effects and a decrease in quality of life, while therapeutic outcomes remain suboptimal.
The limitations of traditional drug delivery methods have prompted research into innovative strategies that enhance the specificity and efficacy of cancer treatments. One such approach is the integration of nanotechnology into drug delivery systems, which allows for the design of nanoparticles that can encapsulate therapeutic agents and improve their distribution within the tumor microenvironment. These nanoparticles can be engineered to respond to specific stimuli or to target tumor-associated antigens, thereby increasing the concentration of drugs at the tumor site while minimizing systemic exposure.
In this study, we present a novel drug delivery strategy that combines microbial synthesis of Prussian blue nanoparticles with a mitochondria-targeting nanoplatform, MiBaMc. This innovative approach not only improves the targeted delivery of therapeutic agents but also harnesses the unique properties of nanomaterials to enhance the efficacy of checkpoint blockade immunotherapy. By addressing the challenges of traditional treatment methods, this research aims to pave the way for more effective and safer cancer therapies, particularly for patients with difficult-to-treat malignancies like TNBC and colorectal cancer.
Research Team and Aim
The research team for this study comprises an accomplished group of scientists led by Dongdong Wang, alongside Jiawei Liu and several collaborators. This research was conducted in 2022 and published in Nature Communications. The team consists of experts from prestigious institutions, including Nanyang Technological University and the University of Science and Technology of China.
The paper, titled “Microbial synthesis of Prussian blue for potentiating checkpoint blockade immunotherapy,” explores innovative strategies to enhance cancer treatment. According to the lead researcher, Dongdong Wang, the aim of the research is to “develop a safe and scalable method for synthesizing Prussian blue nanoparticles that can be used to enhance the effects of immunotherapy in cancer treatment.” This objective underscores the team’s commitment to integrating nanotechnology and immunotherapy to improve therapeutic outcomes for patients afflicted with challenging malignancies.
Experimental Process
Experiment 1: Microbial Synthesis of Prussian Blue Nanoparticles
Primary Technique:
The primary technique employed in this study is microbial synthesis using Shewanella oneidensis MR-1, an electrochemically active bacterium, to produce FDA-approved Prussian blue nanoparticles (PB NPs) through a biological precipitation method.
Key Steps:
- Culturing the Bacteria: S. oneidensis MR-1 was cultivated in Luria-Bertani (LB) medium at 30 °C with shaking until it reached the early exponential growth phase.
- Preparation of Reaction Mixture: In an anaerobic environment, ferric citrate (0.25 M) and potassium hexacyanoferrate (K3[FeIII(CN)6], 0.5 M) were added to the bacterial culture to initiate the synthesis.
- Incubation: The mixture was shaken for 24 hours at 37 °C to allow for the biological precipitation of Prussian blue on the bacterial surfaces.
Data Collection and Analysis:
The synthesis was confirmed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM) to visualize the morphology of the PB nanoparticles. The yield efficiency was calculated by comparing the amount of synthesized PB NPs to the theoretical yield based on the initial concentrations.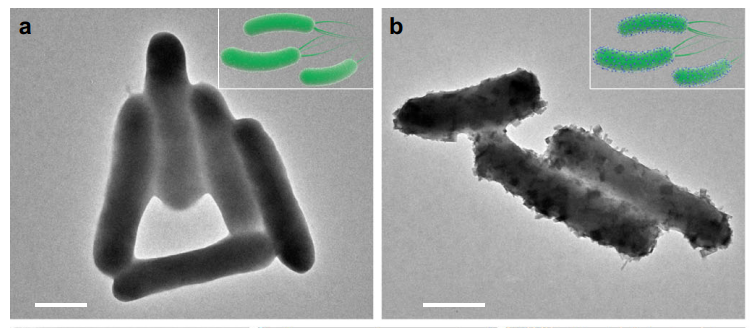
Figure 1. TEM images of a S. oneidensis MR-1 and b S. oneidensis-MOFs. Scale bar, 500 nm.
Result:
The yield efficiency of the synthesized PB NPs was found to be 43.4%, significantly higher than traditional chemical synthesis methods, which exhibited a yield of 21.6%. TEM and SEM images confirmed the successful precipitation of uniform PB nanoparticles on the bacteria.
Novel Aspect:
This experiment demonstrated a novel biological precipitation approach that leverages microbial metabolism for the scalable synthesis of PB NPs, overcoming limitations associated with high temperature and toxic chemicals in traditional chemical synthesis methods.
Experiment 2: Preparation of Mitochondria-Targeting Nanoplatform (MiBaMc)
Primary Technique:
The preparation of the mitochondria-targeting nanoplatform, MiBaMc, involved a two-step modification of the synthesized PB NPs.
Key Steps:
- Fragmentation of Bacterial Membrane: The synthesized S. oneidensis-PB hybrid (S. oneidensis-MOFs) was sonicated for 2 hours to obtain membrane fragments (BaM).
- Conjugation of Chlorin e6 and Triphenylphosphine: BaM was then mixed with chlorin e6 (Ce6) and triphenylphosphine (TPP) in dimethyl sulfoxide (DMSO) under stirring for 1 hour to form the MiBaMc nanoplatform.
Data Collection and Analysis:
High-resolution TEM and energy-dispersive X-ray spectroscopy (EDS) were employed to confirm the successful modification of the PB NPs. Dynamic light scattering (DLS) was used to measure the size distribution of MiBaMc.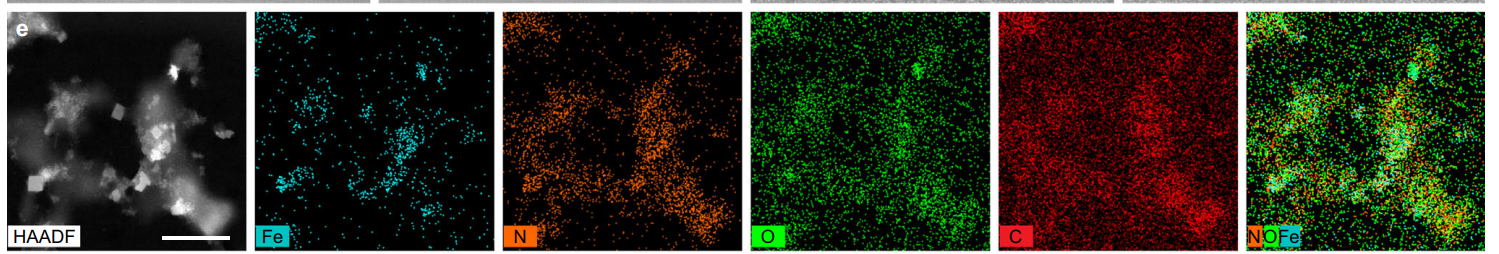
Figure 2. HAADF-STEM image of MiBaMc and corresponding EDS elemental mapping. Scale bar, 200 nm.
Result:
MiBaMc exhibited a uniform particle size with an average diameter of 120 nm, suitable for biological applications. The successful conjugation of Ce6 and TPP was confirmed via UV-Vis spectroscopy, showing characteristic absorption peaks.
Novel Aspect:
The innovative dual-functionalization of PB NPs with Ce6 and TPP allows for enhanced targeting of mitochondria, a feature not typically seen in conventional nano-delivery systems. This targeting capability improves the efficiency of photothermal and photodynamic therapies.
Experiment 3: In Vitro Evaluation of Cytotoxicity and Apoptosis Induction
Primary Technique:
In vitro assays were conducted to assess the cytotoxicity of MiBaMc against 4T1 cancer cells and the induction of apoptosis.
Key Steps:
- Cell Treatment: 4T1 cells were treated with various concentrations of MiBaMc (0, 1, 2, 4, and 8 μg/mL based on Ce6 content) for 24 hours.
- MTT Assay: Cell viability was quantified using the MTT assay to determine the cytotoxic effects of the treatment.
- Apoptosis Analysis: Flow cytometry with Annexin V-FITC/PI staining was performed to quantify apoptotic cells after treatment.
Data Collection and Analysis:
Data from the MTT assay were analyzed using statistical software to evaluate the concentration-dependent effects. Flow cytometry results were analyzed to determine the percentages of live, early apoptotic, and late apoptotic cells.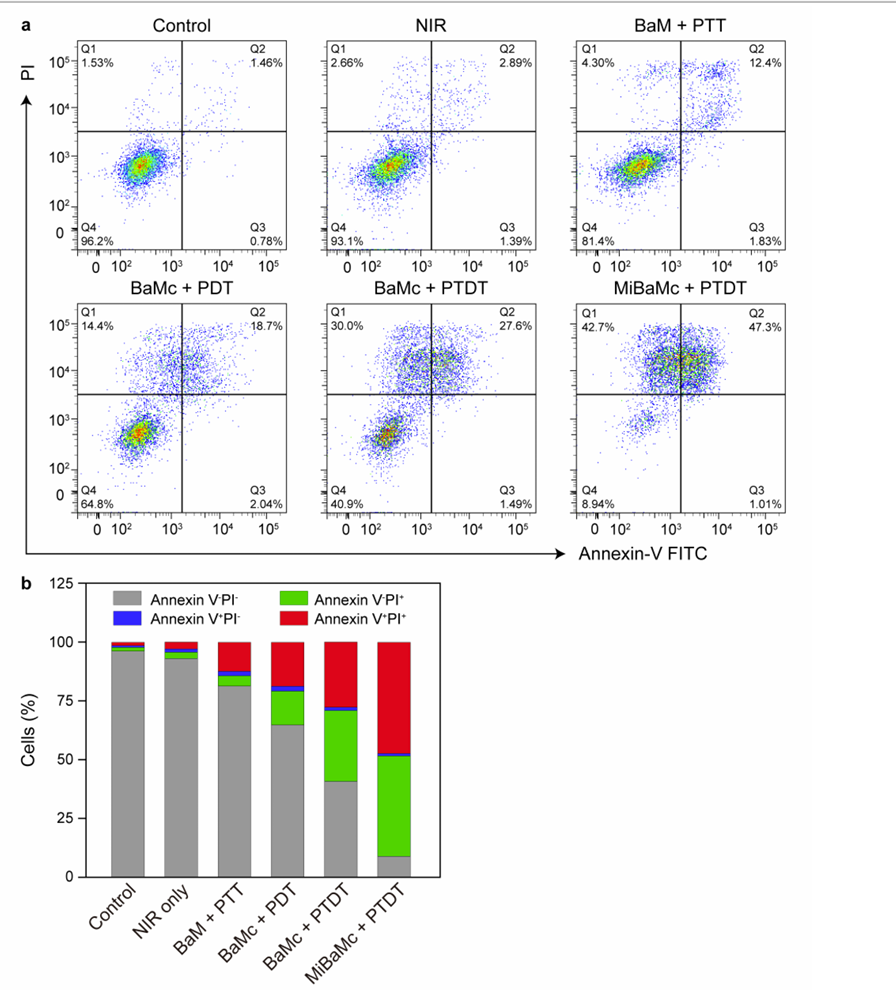
Figure 3. Apoptosis assay 24 h after different treatments.
Result:
MiBaMc exhibited a significant concentration-dependent cytotoxic effect, with an 86.0% killing efficiency at the highest concentration (8 μg/mL). Flow cytometry revealed increased apoptosis in treated cells compared to controls.
Novel Aspect:
This study highlights the enhanced antitumor efficacy of MiBaMc through its ability to induce both photothermal and photodynamic effects, resulting in significant apoptosis rates compared to traditional treatments that often lack such dual-action mechanisms.
Experiment 4: In Vivo Tumor Inhibition Studies
Primary Technique:
In vivo studies were conducted to evaluate the antitumor efficacy of MiBaMc in tumor-bearing mouse models.
Key Steps:
- Tumor Model Establishment: Female Balb/c mice were inoculated subcutaneously with 4T1 cells to establish tumors.
- Treatment Administration: Mice were divided into groups and administered with MiBaMc combined with anti-PDL1 antibody, while control groups received PBS, free Ce6, or single treatments.
- Laser Irradiation: After 12 hours of treatment, the tumors were irradiated with an 808 nm laser for 10 minutes.
Data Collection and Analysis:
Tumor growth was monitored and measured bi-daily using calipers, and the data were statistically analyzed using ANOVA. The final tumor weights were recorded at the study’s endpoint.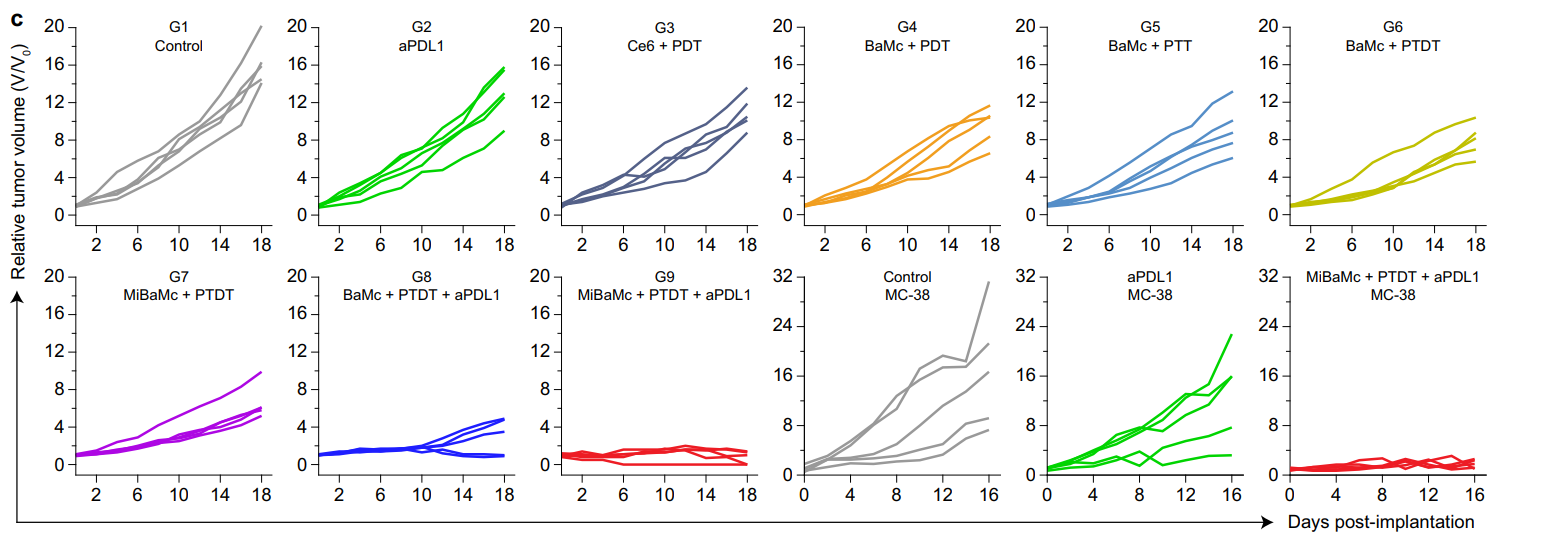
Figure 4. Individual 4T1 and MC38 tumor growth curves during treatment
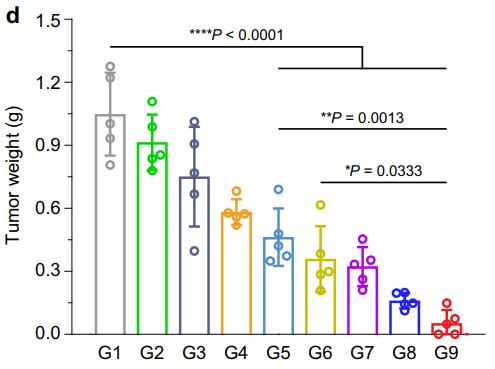
Figure 5. Average 4T1 tumor weight of different groups at the endpoint of treatment (n = 5 mice). Statistical analysis was conducted with ANOVA with Tukey’s tests. * P < 0.05, ** P < 0.01, **** P < 0.0001.
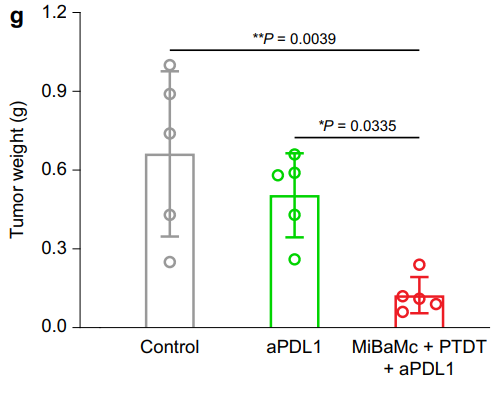
Figure 6. Average MC38 tumor weight of different groups at the endpoint of treatment (n = 5 mice). Statistical analysis was conducted by one-way ANOVA with Tukey’s tests. * P < 0.05, ** P < 0.01.
Result:
Mice treated with MiBaMc combined with anti-PDL1 showed a remarkable 95.5% reduction in tumor volume compared to control groups, with a significant difference in tumor weights observed.
Novel Aspect:
This experiment showcases the potential of combining immunotherapy with a novel nanoplatform, which can significantly enhance therapeutic outcomes in cancer treatment, addressing the low response rates associated with traditional immunotherapies.
Conclusion
The successful development of this drug delivery system was achieved through the innovative integration of microbial synthesis and nanotechnology, which enabled the creation of a mitochondria-targeting nanoplatform, MiBaMc. By utilizing the electrochemically active bacterium Shewanella oneidensis MR-1, the researchers were able to produce FDA-approved Prussian blue nanoparticles through a scalable biological precipitation process. This method not only enhanced the yield and uniformity of the nanoparticles but also facilitated their functionalization with chlorin e6 and triphenylphosphine, allowing for targeted delivery to the mitochondria of cancer cells.
The highlight of the study is the remarkable efficacy demonstrated by MiBaMc in preclinical models, achieving a tumor growth inhibition rate of 95.5% in a triple-negative breast cancer model and 89.4% in a colorectal cancer model when combined with checkpoint blockade immunotherapy. This research not only showcases a novel approach to enhancing cancer immunotherapy but also emphasizes the potential of microbial nanoplatforms to overcome the limitations of traditional treatment methods. The findings pave the way for more effective and safer cancer therapies, particularly for patients facing challenging malignancies.
Reference
Wang, Dongdong, et al. “Microbial Synthesis of Prussian Blue for Potentiating Checkpoint Blockade Immunotherapy.” Nature Communications, vol. 14, no. 2943, 2023, doi:10.1038/s41467-023-38796-9.
