Editor: Sarah
A team of researchers from Shandong University has developed a new drug delivery system that could improve the treatment of Triple-Negative Breast Cancer (TNBC), one of the most challenging and aggressive types of breast cancer. The system is based on a peptide-paclitaxel conjugate, known as PTX-SM-TAR, designed to overcome key limitations associated with the chemotherapy drug paclitaxel (PTX). These challenges include poor solubility in water and non-specific targeting, which limit the drug’s efficacy and increase toxicity. PTX-SM-TAR uses nanoparticles to deliver the drug directly to tumor cells, enhancing both the specificity and penetration into tumor tissues.
Traditional efforts to improve paclitaxel’s effectiveness focused primarily on its solubilization and passive targeting mechanisms. However, this study presents a more advanced approach by utilizing peptide-drug conjugates (PDCs), which enhance both targeting precision and tissue penetration. The innovative aspect of PTX-SM-TAR lies in its incorporation of the TAR peptide, which combines both tumor-targeting and cell-penetrating functions. This development represents a significant step forward in the search for effective treatments for TNBC, a subtype that currently has limited therapeutic options.
The results from animal models were promising, with PTX-SM-TAR demonstrating a significantly higher tumor inhibition rate than paclitaxel alone. In animal trials, PTX-SM-TAR achieved a tumor inhibition rate of 43.24%, compared to 28.47% with paclitaxel alone. These findings underscore the potential of this new system to provide more targeted, efficient, and less toxic chemotherapy for TNBC patients.
Contributions and Key Findings:
- Development of PTX-SM-TAR Conjugate: The research team created a novel conjugate by linking paclitaxel to the TAR peptide using an acid- and esterase-sensitive ester bond. This design ensures that paclitaxel remains stable during circulation but is released in the acidic, enzyme-rich tumor microenvironment.
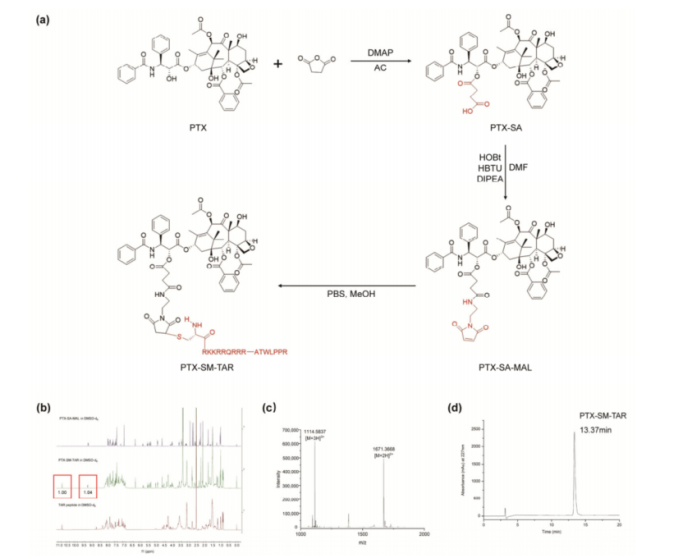
Figure 1: Synthesis and Characterization of PTX-SM-TAR.
- Improved Tumor Targeting and Penetration: The TAR peptide facilitates direct targeting of TNBC cells and enhances the penetration of paclitaxel into tumor tissues. The PTX-SM-TAR nanoparticles were shown to accumulate significantly more in tumor sites compared to paclitaxel alone, while reducing systemic toxicity.
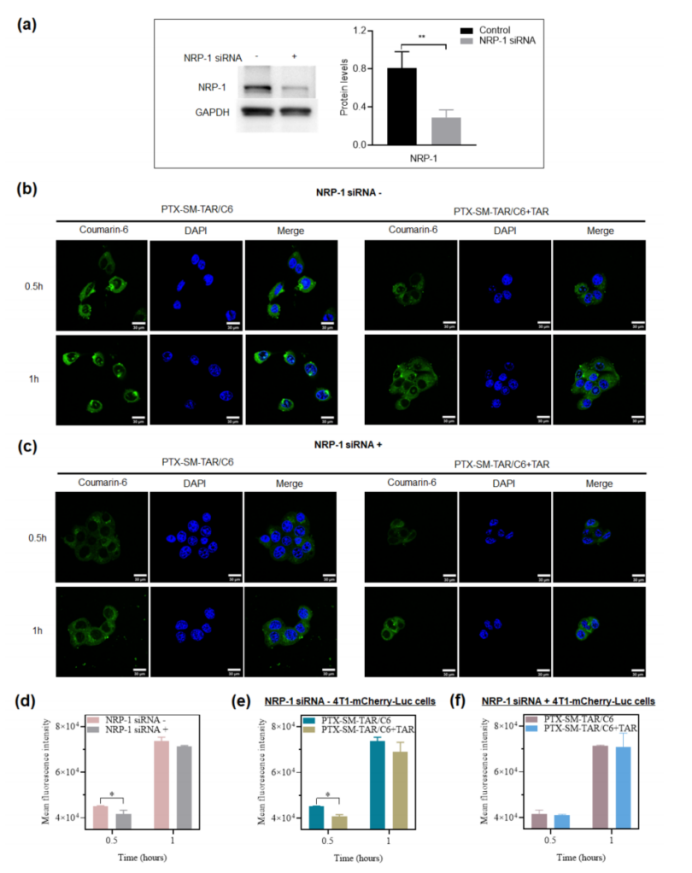
Figure 2: Penetration of PTX-SM-TAR/C6 NPs in the Vascular Barrier In Vitro.
- Nanoparticle Formulation: PTX-SM-TAR self-assembles into nanoparticles that enhance the solubility of paclitaxel. This amphiphilic structure is crucial for improving the delivery and bioavailability of paclitaxel in the body.
- Tumor-Targeting and Receptor-Mediated Endocytosis: In vitro and in vivo tests confirmed that PTX-SM-TAR nanoparticles specifically targeted the Neuropilin-1 (NRP-1) receptor, which is overexpressed in TNBC tumor cells. The TAR peptide plays a crucial role in receptor recognition and facilitating cellular uptake, ensuring more efficient drug delivery to the tumor.
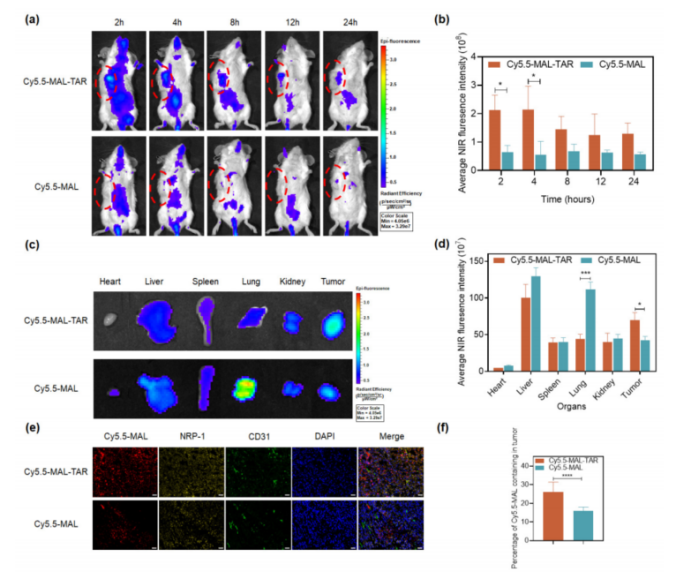
Figure 3: Tumor Targeting Delivery Ability of TAR Peptide.
- Penetration Efficiency: The nanoparticles demonstrated high efficiency in crossing vascular barriers and penetrating deep into tumor tissues. These capabilities were verified through several in vitro experiments, including tumor spheroid models and transcellular migration assays, which showed that PTX-SM-TAR nanoparticles could effectively reach and treat cells deeper within the tumor.
- In Vivo Efficacy: In mouse models of TNBC, PTX-SM-TAR nanoparticles not only demonstrated superior tumor growth inhibition compared to paclitaxel but also showed lower toxicity, as evidenced by the stable body weight of the mice treated with PTX-SM-TAR. The tumors treated with PTX-SM-TAR showed significant apoptosis and tissue damage, as confirmed by histological analyses.
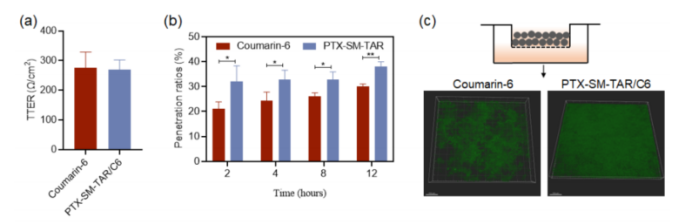
Figure 4: In Vivo Antitumor Efficacy of PTX-SM-TAR NPs on 4T1-mCherry-Luc Xenograft Mice Model.
- Controlled Release Mechanism: The PTX-SM-TAR prodrug exhibited controlled and sustained release of paclitaxel. The ester bond linking paclitaxel to the TAR peptide ensures that the drug is released more efficiently in the tumor environment, where low pH and high esterase activity trigger the breakdown of the conjugate.
Conclusion
This study highlights the promising potential of PTX-SM-TAR as a new, more effective approach to chemotherapy for TNBC. By improving paclitaxel’s solubility, targeting ability, and tumor penetration, this system could offer a more personalized and less toxic treatment option for patients with this aggressive cancer subtype. Furthermore, the results set the stage for future exploration of peptide-drug conjugates in oncology, with potential applications beyond TNBC.
Reference
Wang, Longkun, et al. “Transcytosable Peptide-Paclitaxel Prodrug Nanoparticle for Targeted Treatment of Triple-Negative Breast Cancer.” International Journal of Molecular Sciences, vol. 24, no. 5, 2023, p. 4646. MDPI, https://doi.org/10.3390/ijms24054646. Accessed 27 Apr. 2025.
