Editor: Tiffany
Researchers have developed an ionizable lipid nanoparticle (LNP) platform to deliver TRAIL mRNA directly to the tumor microenvironment, effectively inducing tumor cell death and inhibiting colon cancer progression, especially when combined with tumor microenvironment normalization strategies.
Key Highlights
- Research Question:
Can ionizable lipid nanoparticles effectively deliver TRAIL mRNA to the tumor microenvironment to induce tumor cell death and inhibit colon cancer progression? - Research Difficulties:
The primary challenge was delivering sufficient TRAIL mRNA to the tumor while overcoming the barriers posed by the tumor microenvironment, including vascular compression and extracellular matrix deposition. - Key Findings:
The study demonstrated that LNP-TRAIL effectively induced tumor cell death in vitro and significantly inhibited colon cancer progression in vivo, especially when combined with treatments to normalize the tumor microenvironment. - Innovative Aspects:
This research utilizes a microfluidic mixing process to create lipid nanoparticles that encapsulate mRNA, enhancing the delivery of TRAIL within the tumor. - Importance of the Study:
These findings offer crucial insights into the development of novel therapeutic strategies for solid tumors, potentially leading to more effective cancer immunotherapies.
Colon Cancer: A Global Health Challenge and the Need for Innovative Therapies
Colon cancer represents a significant global health issue, identified as the second leading cause of cancer-related mortality worldwide. In 2023, estimates for the United States projected approximately 2 million new cases and over half a million deaths attributed to this disease. Common symptoms include alterations in bowel habits, rectal bleeding, abdominal discomfort, and unexplained weight loss, all of which impact patient quality of life. Current therapeutic approaches encompass surgery, chemotherapy, radiation, and immunotherapy. Immunotherapy, in particular, has demonstrated potential by augmenting antitumor immune responses with reduced off-target effects. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an immunotherapeutic agent noted for its capacity to selectively trigger apoptosis in cancer cells. However, its clinical application is constrained by a short half-life, off-target effects, and difficulties in achieving effective delivery to the tumor microenvironment (TME). These challenges underscore the necessity for advanced delivery mechanisms to enhance TRAIL’s therapeutic potential.
Developing a Novel LNP Platform for TRAIL mRNA Delivery to the Tumor Microenvironment
The study addressed the question of how to effectively deliver TRAIL to the TME to induce tumor cell apoptosis while mitigating barriers such as abnormal vasculature and dense extracellular matrix within the TME. The objective was to develop an ionizable lipid nanoparticle (LNP) platform for the direct administration of TRAIL mRNA to the TME. The research also aimed to improve delivery efficiency by normalizing the TME using Losartan (Los) or Angiotensin 1-7 (Ang(1-7)), agents known to alleviate vascular compression and extracellular matrix accumulation. This work was conducted by Pedro Pires Goulart Guimaraes and colleagues at the Federal University of Minas Gerais, Brazil, and published in the International Journal of Nanomedicine in 2024.
Evaluating the Efficacy of LNP-TRAIL in Vitro and In Vivo
The investigation employed a systematic experimental framework to evaluate the LNP-TRAIL platform’s efficacy, detailed as follows:
Experimental Procedures
- Formulation of LNP-TRAIL: LNPs encapsulating TRAIL mRNA were prepared using microfluidic mixing techniques.
- Characterization: The LNPs were assessed for size, polydispersity, zeta potential, and pKa to confirm their stability and suitability.
- In Vitro Testing: TRAIL production and tumor cell death induction were examined in cell cultures.
- In Vivo Studies: Mouse models were used to investigate tumor growth inhibition and immune responses post-treatment.
Key Experiments and Findings
Experiment 1: In Vitro Assessment of TRAIL Production and Tumor Cell Death
- Procedure: Colo 205 colon cancer cells and HS-5 stromal cells were exposed to LNP-TRAIL. TRAIL expression was quantified via flow cytometry, and cell viability was measured using a luminescence assay.
- Result: LNP-TRAIL treatment significantly elevated TRAIL production in both cell types. In Colo 205 cells, cell viability was reduced by approximately 38% relative to untreated controls.
- Finding: The LNP-TRAIL system effectively promotes TRAIL-mediated apoptosis in vitro, supporting its viability as a targeted therapeutic approach.
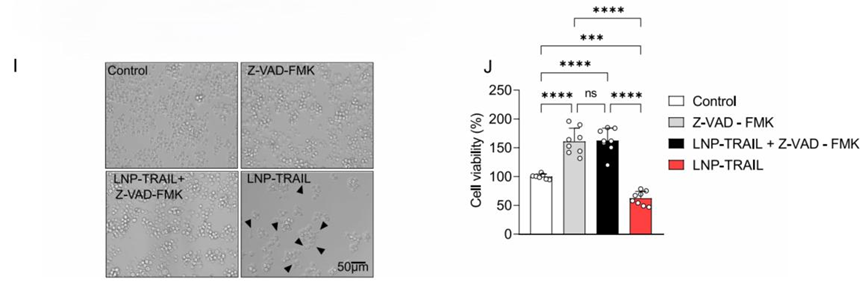
Figure 1. (I) luminescence assay results of Colo 205 cell viability after LNP-TRAIL treatment, with and without Z-VAD-FMK. (J) The significant increase in cell death in LNP-TRAIL-treated cells compared to controls.
Experiment 2: In Vivo Study on Tumor Progression Inhibition with LNP-TRAIL and TME Normalization
- Procedure: Humanized and non-humanized NSG mice bearing Colo 205 tumors received either LNP-TRAIL alone or in combination with Los or Ang(1-7). Tumor volume and weight were tracked over time.
- Result: LNP-TRAIL combined with Los reduced tumor volume by approximately 75%, while combination with Ang(1-7) achieved an 80% reduction, compared to LNP-TRAIL alone.
- Finding: TME normalization with Los or Ang(1-7) substantially enhances the antitumor efficacy of LNP-TRAIL, indicating a synergistic interaction.
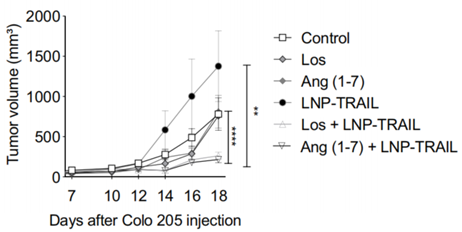
Figure 2. Tumor volume over time in NSG mice, showing a substantial reduction in the LNP-TRAIL plus Los or Ang(1-7) combination treatment compared to LNP-TRAIL alone.
Experiment 3: Analysis of Tumor Cell Death and Immune Cell Infiltration in Humanized Mouse Models
- Procedure: Tumors from humanized mice were evaluated for cell death, caspase-3 activation, and immune cell infiltration using flow cytometry and confocal microscopy.
- Result: Combination treatment increased tumor cell death and caspase-3 activation, alongside elevated infiltration of CD4+ and CD8+ T cells and decreased co-expression of immune checkpoint markers CTLA-4 and PD-1.
- Finding: The combined use of LNP-TRAIL and TME normalization enhances tumor cell apoptosis and bolsters antitumor immune responses, while reducing T-cell exhaustion.
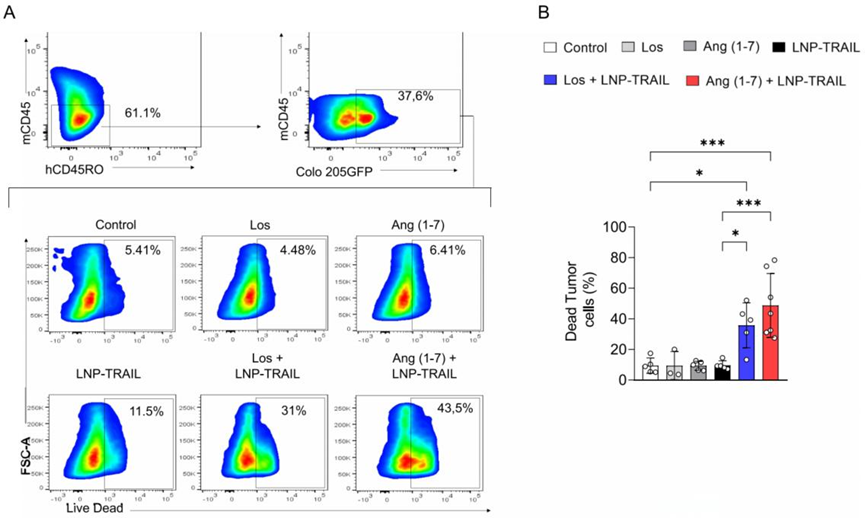
Figure 3. (A) confocal images of tumor sections from humanized mice. (B) the significant rise in tumor cell death in the combination treatment group.
Advancing Colon Cancer Treatment through Targeted mRNA Delivery and TME Normalization
This study introduces an approach to colon cancer therapy through the development of an LNP platform for TRAIL mRNA delivery to the TME. Key outcomes include the successful synthesis of LNP-TRAIL, which exhibited efficacy in both in vitro and in vivo settings. The therapeutic effect was notably amplified when paired with TME normalization using Los or Ang(1-7), achieving up to an 80% reduction in tumor volume and increased immune cell infiltration. These findings highlight the potential of integrating mRNA-based therapies with TME modulation to advance cancer immunotherapy. The research lays groundwork for subsequent studies and clinical evaluations aimed at refining colon cancer treatment strategies.
Reference:
da Silva, Walison Nunes, et al. “Ionizable lipid nanoparticle-mediated TRAIL mRNA delivery in the tumor microenvironment to inhibit colon cancer progression.” International Journal of Nanomedicine (2024): 2655-2673.
