Editor: Nina
Scientists develop a self-augmented ROS-responsive polymeric nanocarrier for synergistic chemoimmunotherapy that co-delivers paclitaxel and 1-methyl-D,L-tryptophan to enhance anti-tumor efficacy and modulate the immunosuppressive tumor microenvironmen.
Key Preview
- Research Question: This study investigates how to improve the efficacy of immunotherapy in cancer treatment, particularly addressing the challenges posed by poor immunogenicity and immunosuppressive tumor microenvironments.
- Research Design and Strategy: The research employs a novel self-augmented reactive oxygen species (ROS)-responsive nanocarrier to co-deliver paclitaxel (PTX), an immunogenic inducer, and 1-methyl-D,L-tryptophan (1-MT), an indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, to enhance tumor rejection.
- Method: The study involved the synthesis of a polymeric nanodrug, characterization of its properties, and in vitro and in vivo evaluations to assess therapeutic efficacy and immune response modulation.
- Key Results: The nanodrug demonstrated significant tumor regression and reduced lung metastasis in 4T1 tumor-bearing mice, with a tumor inhibition rate of 68.3%. The study also showed enhanced infiltration of effector T cells and reduced regulatory T cells and M2-tumor-associated macrophages.
- Significance of the Research: This research highlights a promising approach to overcome immunosuppression in tumors, potentially leading to improved outcomes in cancer immunotherapy.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by the uncontrolled proliferation of abnormal cells that can invade and metastasize to other tissues. Among various types of cancer, triple-negative breast cancer (TNBC) poses a particularly formidable challenge due to its aggressive nature and lack of targeted therapies, contributing to poor prognosis and high recurrence rates. Traditional treatment strategies for cancer often involve the use of chemotherapeutic agents, which aim to kill rapidly dividing tumor cells. However, these treatments can be limited by their systemic toxicity and inability to selectively target tumor tissues, leading to significant side effects and suboptimal therapeutic outcomes.
Commonly, chemotherapeutic drugs are administered through intravenous routes, relying on passive diffusion to reach tumor sites. This conventional approach, while effective in some cases, faces notable challenges, including poor drug solubility, rapid clearance from the bloodstream, and the development of multidrug resistance in tumors. These factors can result in inadequate drug concentrations at the tumor site, necessitating higher doses that exacerbate toxicity and hinder patient compliance. Furthermore, the immunosuppressive tumor microenvironment can undermine the efficacy of chemotherapy by facilitating tumor escape from immune surveillance.
To address these challenges, innovative drug delivery strategies have emerged, focusing on the development of nanocarrier systems that enhance the delivery and efficacy of therapeutic agents. One promising approach is the use of self-augmented reactive oxygen species (ROS)-responsive polymeric nanocarriers, which can achieve targeted delivery of chemotherapeutics while simultaneously modulating the immune response. By encapsulating immunogenic inducers and immune modulators within a single nanocarrier, this innovative strategy seeks to enhance tumor immunogenicity, overcome the immunosuppressive microenvironment, and improve overall therapeutic outcomes in cancer treatment. This study explores the potential of such a nanocarrier system, combining paclitaxel (PTX), an established chemotherapeutic agent, with 1-methyl-D,L-tryptophan (1-MT), an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), to synergistically enhance anti-tumor efficacy and immune response.
Research Team and Aim
The research was conducted by a team of experts in the field, led by Jinxiao Song, alongside Mingyang Cheng, Yi Xie, Kangkang Li, and Xinlong Zang. The research was carried out at the School of Basic Medicine at Qingdao University. The study, titled “Efficient tumor synergistic chemoimmunotherapy by self-augmented ROS-responsive immunomodulatory polymeric nanodrug,” was published in the Journal of Nanobiotechnology in 2023.
The aim of the research, as articulated by the lead researcher, was to develop a polymeric nanocarrier that enhances the efficacy of chemoimmunotherapy by co-delivering paclitaxel (PTX) and 1-methyl-D,L-tryptophan (1-MT), targeting both tumor cells and the immunosuppressive tumor microenvironment. The research seeks to address the dual challenges of enhancing tumor immunogenicity while concurrently modulating the immunosuppressive conditions typically found in tumors, thereby improving therapeutic outcomes in cancer treatment.
Experimental Process
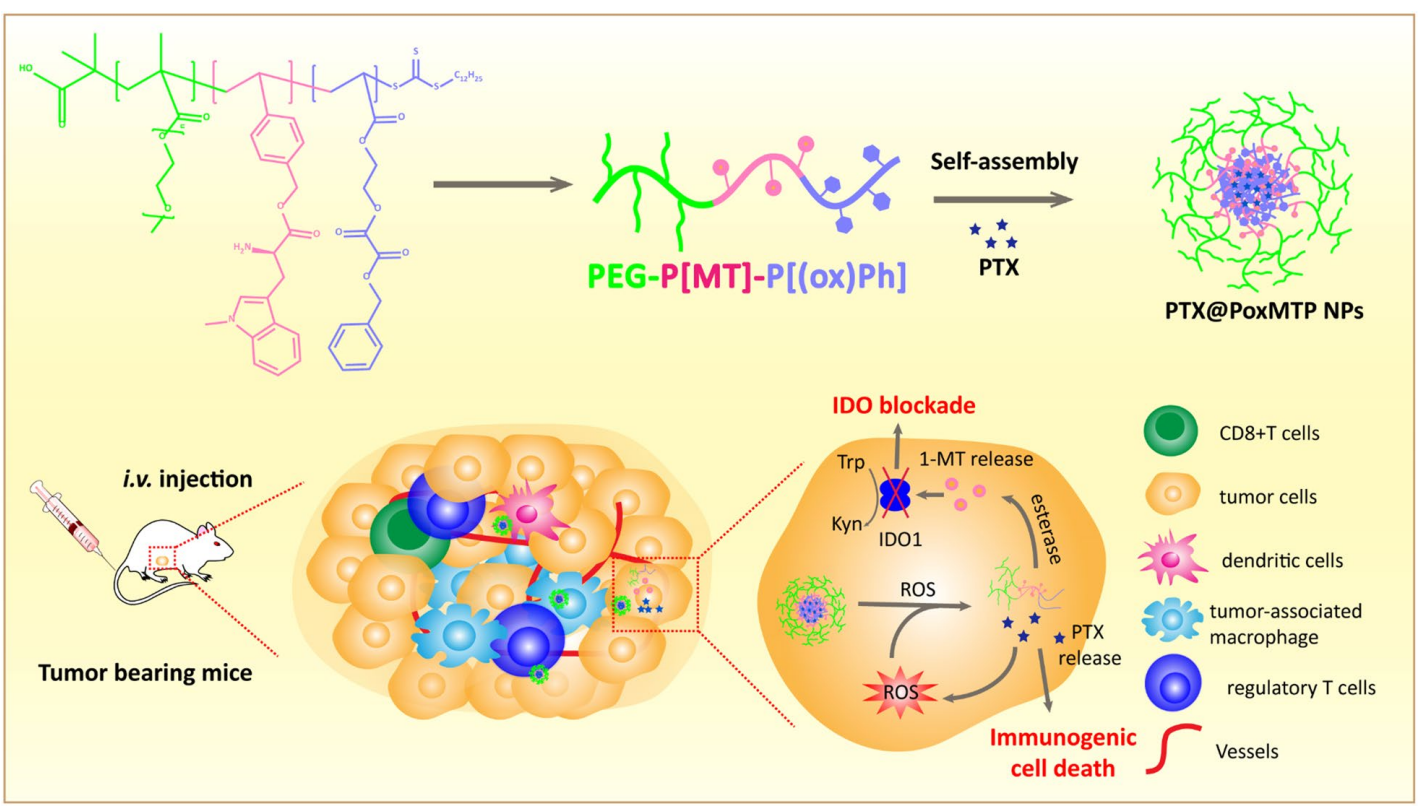
Scheme 1. Schematic illustration of the formation of ROS-augmented nanocarrier driven by self-assembly in aqueous solution. PTX-mediated ROS generation further “fuels” nanoparticle disassembly and PTX release to promote tumor immunogenicity. Meanwhile esterase responsive 1-MT release intervenes IDO mediated immunosuppressive tumor microenvironment
1. Synthesis of the Polymeric Nanodrug
Primary Technique: The primary technique employed in this study is the synthesis of a self-augmented reactive oxygen species (ROS)-responsive polymeric nanodrug through a reversible addition-fragmentation chain transfer (RAFT) polymerization method.
Key Steps:
- Monomer Preparation: 1-Methyl-D, L-tryptophan (1-MT) was protected with di-tert-butyl dicarbonate (Boc) through an amidation reaction. This was followed by the synthesis of phenyl-ox-acrylate by reacting phenylmethanol with 2-hydroxyethyl methacrylate (2-HEMA) in the presence of oxalyl chloride.
- Polymerization: The prepared monomers (1-MT(Boc) acrylate, phenyl-ox-acrylate, and poly(ethylene glycol) methacrylate) were combined in a Schlenk tube with the RAFT initiator 2-(dodecylthiocarbonothioylthio) propionic acid (DoPAT) and AIBN, then subjected to triple freeze-pump-thaw cycles to remove oxygen before stirring at 90 °C for 5 hours.
- Purification: The resultant copolymer was purified via precipitation in cold ether to obtain the final polymeric product.
Data Collection and Analysis: The successful synthesis of the polymer was confirmed using proton nuclear magnetic resonance (1H NMR) spectroscopy, and the hemolytic activity was measured to assess biocompatibility.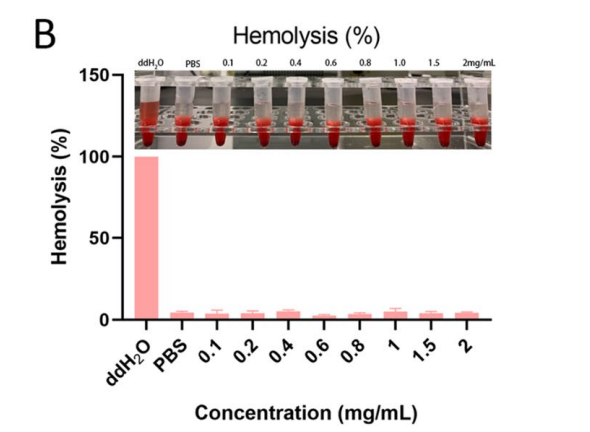
Figure 1. Hemolytic assay of the copolymer
Result: The synthesized polymer demonstrated a successful incorporation of 1-MT and phenyl-ox-acrylate, achieving a polymer ratio of PEG: P[MT]: P[Ph(ox)] of 2:1.1:1.1, along with negligible hemolytic activity (~4%) at concentrations below 2 mg/mL.
Novel Aspect: This study innovatively combines the properties of ROS responsiveness with the immunomodulatory capabilities of IDO1 inhibitors within a single polymeric nanocarrier. This dual-functionality enhances the therapeutic potential over traditional nano-delivery systems that often deliver singular or non-responsive therapeutics.
2. Preparation of PTX@PoxMTP NPs
Primary Technique: The preparation of paclitaxel-loaded polymeric nanoparticles (PTX@PoxMTP NPs) was achieved using the thin-film hydration method.
Key Steps:
- Film Formation: The synthesized polymer (P[Ph(ox)]-P[MT]-PEG) and PTX were dissolved in dichloromethane (DCM) and subjected to rotary evaporation at 35 °C to form a thin film.
- Hydration: The thin film was then hydrated with phosphate-buffered saline (PBS) at 60 °C and subjected to sonication for 10 minutes to facilitate nanoparticle formation.
- Filtration: The resultant suspension was filtered through a 0.22 µm filter to remove any unencapsulated drug.
Data Collection and Analysis: Dynamic light scattering (DLS) was employed to measure particle size and polydispersity index (PDI), while transmission electron microscopy (TEM) was used for morphological assessment.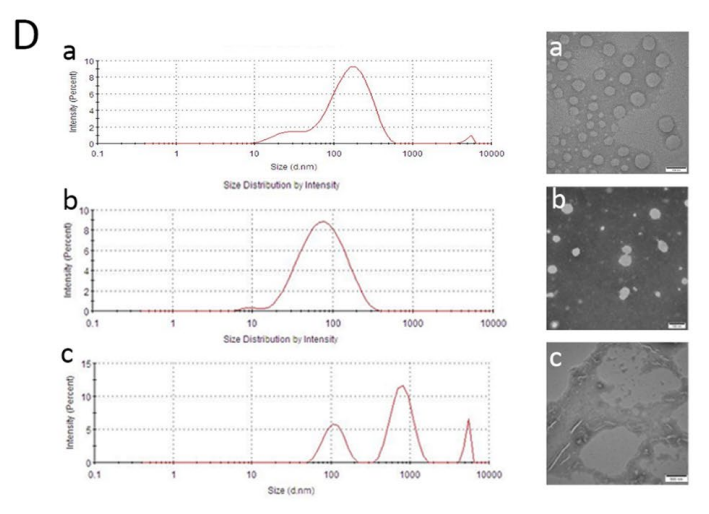
Figure 2. (D) Particle size distribution of blank (a), PTX loaded (b) and H2O2 treated nanoparticles © by DLS and TEM, scale bar = 100 nm
Result: The resulting PTX@PoxMTP NPs exhibited an average diameter of 62.09 nm with a PDI of 0.264, confirming their optimal size for in vivo delivery. Encapsulation efficiency was observed at 62.5%, demonstrating a successful loading of PTX.
Novel Aspect: The combination of ROS-responsive release and the simultaneous delivery of an IDO1 inhibitor offers a significant advancement in targeting tumor microenvironments compared to traditional systems that lack this dual-action capability.
3. In Vitro Cytotoxicity Assay
Primary Technique: MTT assay was utilized to evaluate the cytotoxicity of the PTX formulations on MDA-MB-231 and 4T1 cancer cell lines.
Key Steps:
- Cell Seeding: Cells were seeded in 96-well plates at a density of 1 × 10^4 cells per well and allowed to adhere overnight.
- Treatment: The cells were treated with various concentrations of PTX, PoxMTP NPs, and PTX@PoxMTP NPs for 24 hours.
- MTT Assay: Following treatment, 10 µL of MTT solution was added to each well, incubated for 4 hours, and then dissolved in DMSO to measure absorbance at 570 nm.
Data Collection and Analysis: Cell viability was calculated using the formula:
[ \text{Cell Survival (%)} = \frac{\text{Atreated – Ablank}}{\text{Acontrol – Ablank}} \times 100 ]
IC50 values were determined using GraphPad Prism software.
Result: PTX@PoxMTP NPs significantly reduced cell viability with IC50 values of 2.134 µg/mL for MDA-MB-231 and 2.053 µg/mL for 4T1 cells, indicating enhanced cytotoxicity compared to free PTX.
Novel Aspect: The enhanced cytotoxicity of PTX@PoxMTP NPs underscores the effectiveness of the ROS-responsive delivery system in achieving higher drug concentrations at the target site, which is often a limitation in conventional drug formulations.
4. In Vivo Biodistribution Study
Primary Technique: In vivo imaging was performed using DiR-labeled nanoparticles to assess biodistribution in 4T1 tumor-bearing mice.
Key Steps:
- Tumor Model Establishment: Female Balb/c mice were subcutaneously injected with 1 × 10^7 4T1 cells to establish tumor xenografts.
- Injection of Nanoparticles: Once tumors reached approximately 100 mm³, mice were intravenously administered with DiR-labeled PTX@PoxMTP NPs.
- Imaging: Biodistribution was monitored at various time points using an in vivo imaging system, and major organs were excised for ex vivo imaging after 48 hours.
Data Collection and Analysis: Fluorescence imaging was used to quantify the accumulation of nanoparticles in the tumor and organs, allowing the evaluation of targeted delivery.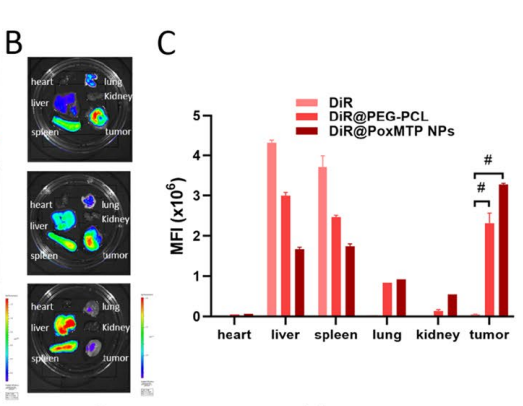
Figure 3. B–C Fluorescence images and analysis of excised major organs and tumors at 48 h after intravenous administration.
Result: DiR-labeled nanoparticles showed significant accumulation in the tumor site by 4 hours post-injection, indicating effective tumor targeting and reduced distribution to non-target organs.
Novel Aspect: The use of a ROS-responsive system allows for enhanced retention and accumulation at the tumor site, contrasting with traditional nanoparticle formulations that often distribute non-specifically, leading to increased systemic toxicity.
5. Immune Response Analysis
Primary Technique: Flow cytometry was utilized to evaluate immune cell infiltration in tumor tissues post-treatment.
Key Steps:
- Tumor Harvesting: After treatment with PTX@PoxMTP NPs, tumors were harvested and digested in a collagenase/DNase solution to obtain a single-cell suspension.
- Staining: The resulting cell suspensions were stained with fluorescent antibodies targeting CD8+, regulatory T cells (Tregs), and dendritic cells (DCs).
- Flow Cytometry: Samples were analyzed using a flow cytometer to quantify the populations of immune cells.
Data Collection and Analysis: The percentage of infiltrating CD8+ T cells, Tregs, and DCs were calculated and compared across treatment groups.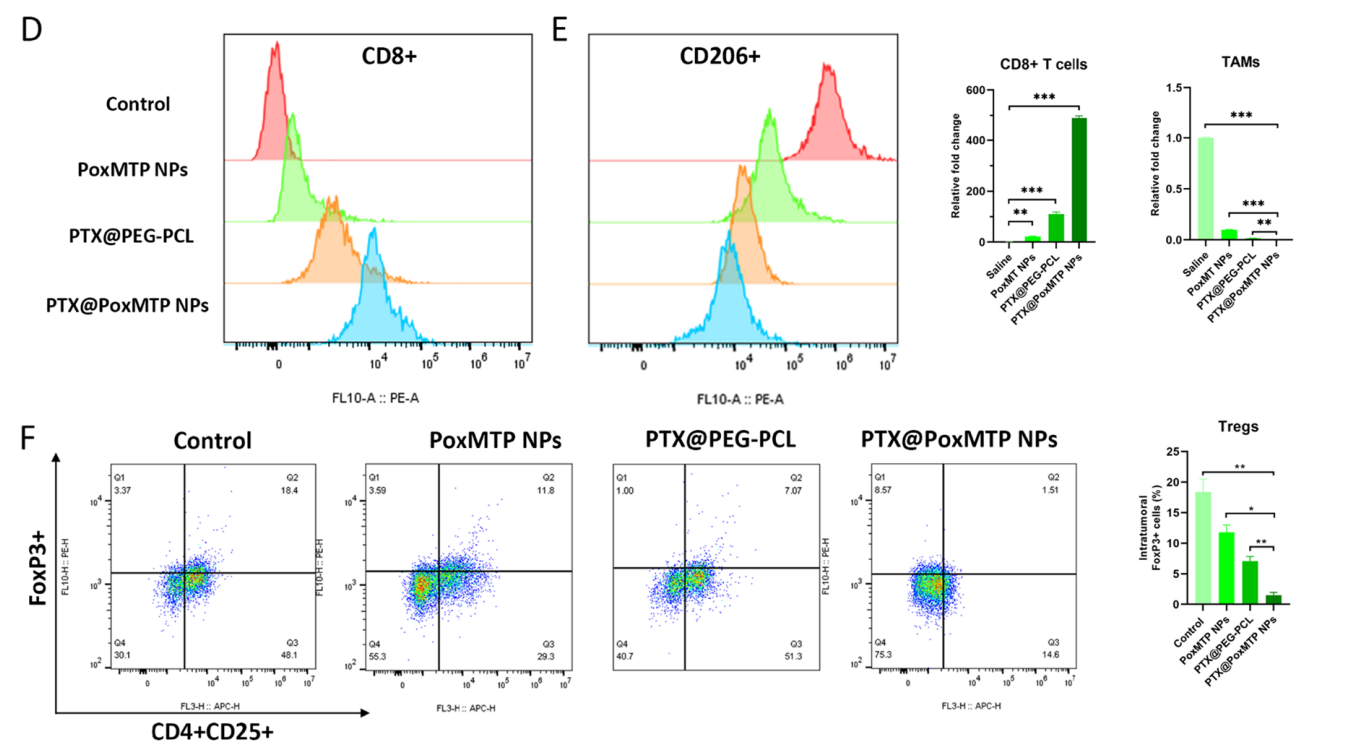
Figure 4. D–F Flow cytometry and analysis of CD8 + T cells, TAMs and Tregs infltration in tumor cells. *P < 0.05, **P < 0.01 and ***P < 0.001
Result: PTX@PoxMTP NPs significantly increased CD8+ T cell infiltration and reduced Treg levels, indicating a favorable shift in the tumor microenvironment towards enhanced anti-tumor immunity.
Novel Aspect: This study highlights the ability of the ROS-responsive nanocarrier to modulate the immune landscape of tumors, promoting an effective immune response while simultaneously reducing immunosuppressive Treg populations, which is a critical advancement over traditional therapies that do not address immune modulation.
Conclusion
The study successfully developed a self-augmented ROS-responsive nanocarrier that enhances the efficacy of chemoimmunotherapy by delivering PTX and 1-MT. Key findings revealed that this dual-delivery system not only promoted immunogenic cell death but also modulated the immunosuppressive tumor microenvironment, resulting in a robust anti-tumor immune response. Despite its promising results, the study acknowledges limitations such as the need for further testing in clinical settings and potential variations in response among different cancer types. Future research should explore optimizing the formulation and dosage to maximize therapeutic benefits while minimizing side effects. Overall, this innovative approach offers a significant advancement in the field of cancer therapy, potentially improving outcomes for patients facing aggressive tumors.
Reference
Song, Jinxiao, et al. “Efficient Tumor Synergistic Chemoimmunotherapy by Self-Augmented ROS-Responsive Immunomodulatory Polymeric Nanodrug.” Journal of Nanobiotechnology, vol. 21, no. 93, 2023, https://doi.org/10.1186/s12951-023-01842-1.
