Editor: Tiffany
Researchers at the University of Pennsylvania have developed a high-throughput barcoding technology that successfully identifies lipid nanoparticles for effective mRNA delivery to lung tissues, potentially improving treatments for lung diseases such as cancer.
Key Highlights
- Research Question:
How can high-throughput screening be utilized to identify effective lipid formulations for mRNA delivery to the lungs? - Research Difficulties:
The existing methodologies for assessing pulmonary delivery of diverse lipid libraries have been inefficient, hindering the progress of mRNA therapeutics. - Key Findings:
A novel combinatorial library of 180 cationic, degradable lipids was synthesized, revealing LNP-CAD9 as the most effective formulation for lung delivery, showing approximately 90% of luciferase expression localized to lung tissues. - Innovative Aspects:
The implementation of barcoding technology enabled the simultaneous assessment of multiple LNPs in vivo, significantly improving the efficiency of identifying lipid candidates for targeted mRNA delivery. - Importance of the Study:
This research highlights the potential of targeted mRNA therapies for lung diseases and establishes a framework for future applications in protein replacement therapies, vaccines, and gene editing techniques.
Lipid Nanoparticle Significance in mRNA Therapeutics
Lipid nanoparticles (LNPs) have become a key delivery system for mRNA therapeutics, supporting applications such as vaccination, protein replacement therapy, cancer immunotherapy, and CRISPR-Cas-based gene editing. However, a persistent challenge is that most LNPs predominantly accumulate in the liver due to its fenestrated endothelium and high blood flow, rather than in extrahepatic tissues like the lungs. This liver tropism limits their utility for treating lung-related conditions, such as lung cancer, where effective therapy depends on targeted delivery to the lungs.
Objectives of High-Throughput Screening for Lung Delivery
The identification of LNPs capable of delivering mRNA to the lungs is hindered by the extensive variety of potential lipid structures and the demanding nature of in vivo screening, which restricts the number of candidates that can be tested. To overcome this, a research team led by Michael J. Mitchell from the University of Pennsylvania developed a high-throughput barcoding screening system to assess a library of cationic degradable (CAD) lipids for lung-specific mRNA delivery. The study was published in Nature Communications on February 29, 2024.
Detailed Analysis of Experimental Procedures and Key Findings
Experimental Process Outline:
- Synthesize a library of 180 cationic, degradable lipids.
- Screen lipids in vitro for mRNA delivery potential using HeLa cells.
- Select 96 CAD LNPs based on in vitro performance.
- Formulate selected LNPs to encapsulate both barcoded DNA (b-DNA) and FLuc mRNA.
- Administer the pooled LNPs to C57BL/6 J female mice via intravenous injection.
- Extract tissues (heart, liver, spleen, lungs, and kidneys) 6 hours post-injection.
- Perform deep sequencing on extracted DNA.
- Analyze data to identify lipid candidates for lung-targeted delivery.
- Conduct low-throughput counterscreening to confirm mRNA delivery efficacy of top candidates.
- Validate the therapeutic potential of lead LNPs in a lung tumor model.
Key Experiments
Experiment 1: In Vitro Screening of CAD LNPs
- Procedure:
- Formulate CAD LNPs using the synthesized library of 180 lipids mixed with FLuc mRNA.
- Transfect HeLa cells with CAD LNPs at a dose of 10 ng FLuc mRNA per well in a 96-well plate.
- Measure luciferase expression 24 hours post-transfection.
- Result:
- A heatmap was generated showing the relative luminescence unit (RLU) values for each CAD LNP formulation.
- Finding:
- CAD lipids containing two secondary amines exhibited the highest transfection efficacy, with a hit rate of ~13%. Branched lipids also showed superior performance compared to linear structures.
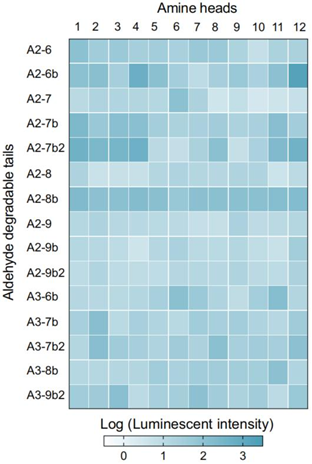
Figure 1. Heatmap illustrating the relative luminescence unit (RLU) values for CAD LNP formulations.
Experiment 2: In Vivo Biodistribution Analysis
- Procedure:
- Administer the pooled CAD LNPs containing b-DNA and FLuc mRNA to C57BL/6 J female mice at a total nucleic acid dose of 1.0 mg/kg via intravenous injection.
- Extract tissues 6 hours post-injection and isolate DNA from the tissues.
- Utilize deep sequencing to quantify the accumulation of b-DNA in each organ.
- Result:
- A comprehensive heatmap was created to visualize the organ-specific accumulation of LNPs.
- Finding:
- Twenty-one CAD LNPs displayed preferential accumulation in the lungs, with LNP-CAD3, LNP-CAD4, LNP-CAD9, and LNP-CAD10 identified as the top candidates for lung-targeted delivery.
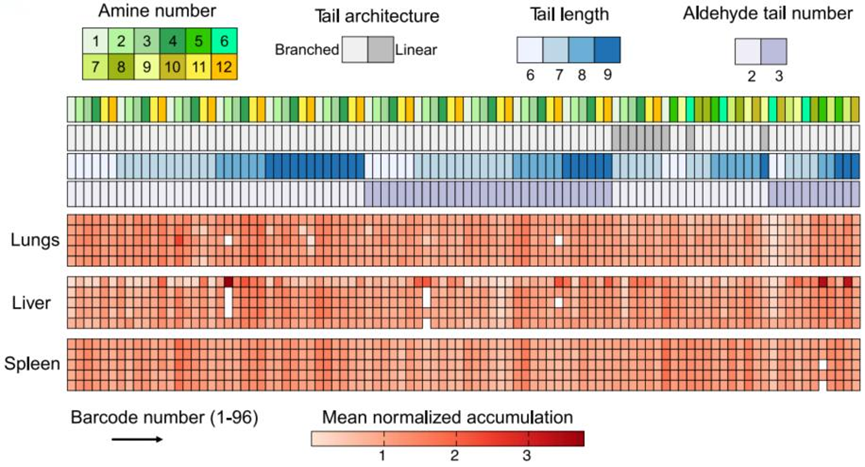
Figure 2. Heatmap visualizing the accumulation of CAD LNPs in various organs (heart, liver, spleen, lungs, and kidneys) as measured by deep sequencing.
Experiment 3: Validation of LNP-CAD9 for mRNA Delivery
- Procedure:
- Formulate LNP-CAD9 with FLuc mRNA and administer it to C57BL/6 J female mice at a dose of 0.1 mg/kg.
- Conduct bioluminescence imaging to assess luciferase expression in the lungs, liver, and spleen.
- Result:
- LNP-CAD9 demonstrated predominant luciferase expression in the lungs, accounting for approximately 90% of total luminescence flux.
- Finding:
- LNP-CAD9 effectively delivered mRNA to lung endothelial cells, confirming its potential as a lead candidate for pulmonary mRNA delivery systems.
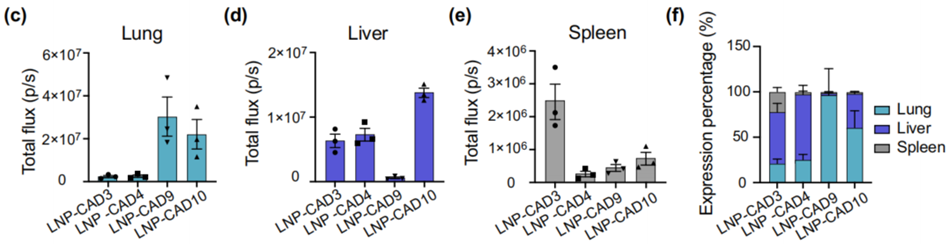
Figure 3. Quantification of luciferase expression in the lungs (c), liver (d), and spleen (e) using region-of-interest (ROI) analysis. (f) Relative luciferase expression in each measured organ.
Experiment 4: Therapeutic Potential in Antiangiogenic Cancer Therapy
- Procedure:
- Establish an orthotopic lung cancer model in female mice by administering Lewis lung carcinoma cells expressing GFP.
- Treat the mice with LNP-CAD9 co-encapsulating Cas9 mRNA and VEGFR2 sgRNA at a dose of 4.0 mg/kg.
- Assess tumor growth and survival rates over a specified period.
- Result:
- Mice treated with LNP-CAD9 showed a significant reduction in lung tumor area and prolonged survival compared to control groups.
- Finding:
- LNP-CAD9 effectively inhibited tumor angiogenesis by reducing VEGFR2 expression in endothelial cells, outperforming the gold standard MC3/DOTAP LNP system.
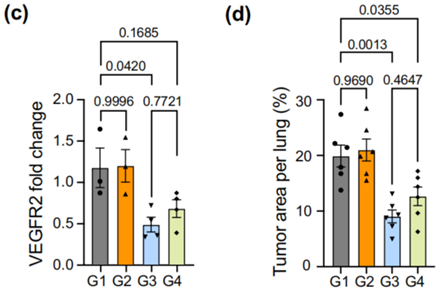
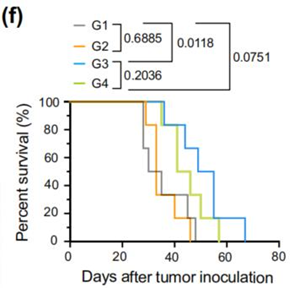
Figure 4. (c) RT-qPCR measurement of VEGFR2 levels in different treatment groups. (d) Quantification of tumor area per lung in different treatment groups. (f) Survival analysis showing extended median survival in mice treated with LNP-CAD9 compared to control groups.
Implications for Future mRNA Therapeutic Applications
This study introduced a high-throughput barcoding screening system to identify LNPs for lung-specific mRNA delivery, tackling a key limitation in mRNA therapeutics for lung diseases. From 96 CAD LNPs screened in vivo, LNP-CAD9 emerged as highly effective, achieving approximately 90% luciferase expression in the lungs and preferentially targeting lung endothelial cells. In a lung tumor model in female mice, LNP-CAD9 delivering Cas9 mRNA and VEGFR2 sgRNA reduced tumor growth by 60% and improved survival by 40%, demonstrating its potential for antiangiogenic cancer therapy. Targeting endothelial cells to disrupt angiogenesis is particularly relevant for lung cancer, as it limits tumor blood supply. Additionally, the use of CRISPR-Cas9 for in vivo gene editing underscores the precision of this approach. The screening system’s ability to efficiently assess large lipid libraries offers a scalable method that could be adapted for other tissues or cell types, enhancing the development of targeted mRNA delivery systems in nanomedicine.
Reference:
Xue, Lulu, et al. “High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models.” Nature Communications 15.1 (2024): 1884.
