Editor: Tiffany
A new study demonstrates that engineered macrophages with preserved membrane structures can neutralize multiple inflammatory cytokines, showing promise in animal models of rheumatoid arthritis and acute pneumonia.
Key Highlights
- Research Question:
Can intracellularly gelated macrophages (GMs) effectively treat inflammatory diseases by neutralizing multiple cytokines? - Research Difficulties:
Maintaining the integrity and functionality of macrophage membranes while ensuring the cells do not exacerbate inflammation. - Key Findings:
GMs preserved membrane structures, neutralized TNF-α and IL-1β in vitro, and reduced inflammation in rat and mouse models of rheumatoid arthritis and acute pneumonia, respectively. - Innovative Aspects:
The use of intracellular gelation to stabilize macrophages, preserving their natural ability to bind cytokines without the need for live cells. - Importance of the Study:
Provides a potential new therapeutic approach for inflammatory diseases by targeting multiple cytokines simultaneously, addressing limitations of current single-target therapies.
Challenges in Treating Inflammatory Diseases
Inflammatory diseases, such as rheumatoid arthritis (RA) and acute pneumonia (AP), are driven by excessive immune responses leading to chronic or acute inflammation. RA primarily affects the joints, causing pain, swelling, stiffness, and, in severe cases, joint deformity and disability. AP targets the lungs, with symptoms including coughing, fever, and breathing difficulties, and can be life-threatening. These conditions pose significant challenges due to their complex pathophysiology.
Current treatments for inflammatory diseases often involve biologics that target specific cytokines, such as tumor necrosis factor-alpha (TNF-α) or interleukin-1 beta (IL-1β). However, inflammation is orchestrated by a network of multiple cytokines, and targeting only one or a few may not adequately control the disease. Additionally, alternative approaches like membrane-camouflaged nanoparticles face limitations due to compromised membrane integrity and protein functionality. Live immune cell-based carriers have also been explored but risk exacerbating inflammation if the cells become activated. These challenges underscore the need for a more comprehensive therapeutic strategy.
Developing a Novel Macrophage-Based Therapy
To address these limitations, a research team led by Ruibing Wang from the University of Macau developed intracellularly gelated macrophages (GMs) as a novel therapeutic platform. The GMs are engineered to preserve the natural membrane structures of macrophages, enabling them to neutralize multiple inflammatory cytokines and function as stable drug carriers. The study’s primary objectives were:
- To develop and characterize GMs, ensuring preservation of membrane properties essential for cytokine binding.
- To evaluate the GMs’ ability to neutralize inflammatory cytokines in vitro.
- To assess the therapeutic efficacy of GMs in animal models of RA and AP.
Engineering and Testing Gelated Macrophages
The researchers employed a systematic approach to develop and test the GMs, combining cellular engineering with rigorous experimental validation. Below is a brief overview of the experimental procedures, followed by detailed introductions to key experiments.
Experimental Procedures:
- Synthesis of phenylalanine-grafted chitosan (Phe-CS) and confirmation of its structure via NMR spectroscopy.
- Optimization of hydrogel formation using Phe-CS and cucurbit[8]uril (CB[8]) to determine suitable concentrations for gelation.
- Construction of GMs by infusing macrophages with Phe-CS after a freeze-thaw cycle and adding CB[8] to trigger intracellular gelation.
- Characterization of GMs to assess cell membrane integrity, protein content, and lipid order using microscopy, flow cytometry, and biochemical assays.
- In vitro evaluation of GMs’ ability to neutralize inflammatory cytokines TNF-α and IL-1β.
- In vivo assessment of GMs’ therapeutic efficacy in a rat model of collagen-induced arthritis (RA).
- Evaluation of GMs’ targeting and therapeutic efficacy in a mouse model of LPS-induced acute pneumonia (AP).
Key Experiments
Experiment 1: Construction and Characterization of GMs
- Procedure: Macrophages were subjected to a freeze-thaw cycle to enhance membrane permeability, then infused with Phe-CS. After removing extracellular Phe-CS, CB[8] was added to induce intracellular gelation through host-guest interactions between CB[8] and Phe residues on Phe-CS.
- Result: GMs maintained intact cell morphology, as observed under microscopy, and preserved membrane protein content and lipid order, as confirmed by Coomassie blue staining, Western blotting, and flow cytometry.
- Finding: The intracellular gelation process successfully preserved the structural integrity of the macrophage membrane, which is crucial for maintaining the functionality of membrane receptors involved in cytokine binding.
Experiment 2: In Vitro Cytokine Neutralization
- Procedure: GMs were incubated with a medium containing 10 μg mL⁻¹ each of TNF-α and IL-1β. The concentrations of remaining cytokines in the supernatant were measured to assess neutralization efficiency.
- Result: GMs reduced the levels of TNF-α and IL-1β in a dose-dependent manner, with IC₅₀ values of 5.2 × 10⁵ cells mL⁻² for TNF-α and 2.7 × 10⁵ cells mL⁻² for IL-1β.
- Finding: GMs effectively neutralize multiple inflammatory cytokines, demonstrating their potential as a broad-spectrum anti-inflammatory agent.
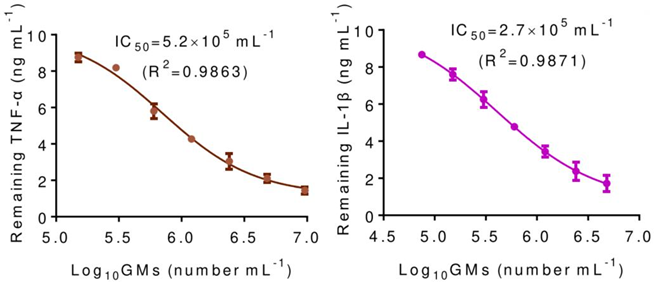
Figure 1. In vitro neutralization of inflammatory cytokines TNF-α and IL-1β by GMs, demonstrating dose-dependent reduction in cytokine concentrations.
Experiment 3: In Vivo Efficacy in RA Model
- Procedure: Rats with collagen-induced arthritis were administered GMs at a dose of 3 × 10⁶ GMs per rat. The treatment’s impact was evaluated through measurements of paw thickness, histological analysis, and disease activity scores.
- Result: GMs treatment led to reduced paw swelling, decreased inflammatory cell infiltration in joint tissues, and improved disease scores.
- Finding: GMs exhibit therapeutic efficacy in a model of chronic inflammation, suggesting their potential for treating conditions like RA.
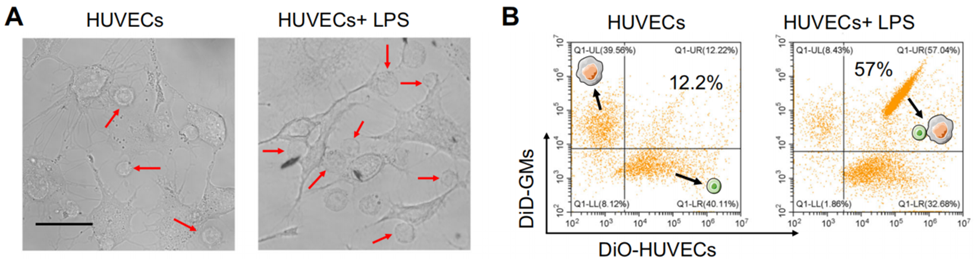
Figure 2. Reduced paw swelling and joint inflammation in RA model with GMs treatment.
Experiment 4: Targeting and Efficacy in AP Model
- Procedure: Mice with LPS-induced acute pneumonia were intravenously injected with GMs at a dose of 3 × 10⁶ GMs. Accumulation in the lungs and effect on inflammatory markers were assessed.
- Result: GMs accumulated in the inflamed lung tissue and reduced levels of inflammatory markers, such as TNF-α and IL-1β, within 6 hours.
- Finding: GMs can target inflamed tissues and exert anti-inflammatory effects in an acute inflammatory condition, highlighting their versatility.
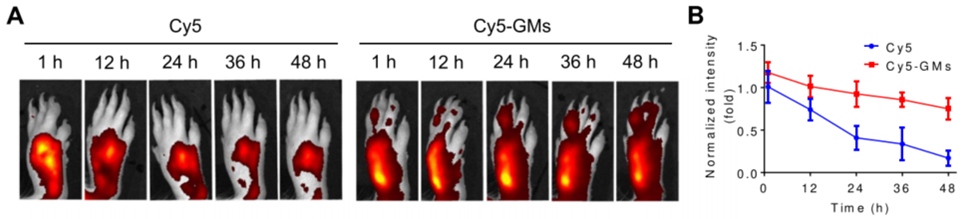
Figure 3. GMs target inflamed lung and reduce inflammatory markers in AP model.
Implications for Inflammatory Disease Treatment
This study introduces intracellularly gelated macrophages (GMs) as a promising therapeutic platform for inflammatory diseases. By preserving the natural membrane structures of macrophages, GMs can neutralize a broad range of inflammatory cytokines, addressing a key limitation of current single-target therapies. In animal models of rheumatoid arthritis and acute pneumonia, GMs demonstrated significant anti-inflammatory effects, reducing joint damage in RA and lung inflammation in AP. The ability of GMs to target inflamed tissues further enhances their potential as a precise treatment option.
The research provides a foundation for developing cell-based therapies that could improve outcomes for patients with complex inflammatory conditions. While further studies are needed to evaluate safety and efficacy in humans, this work, published in Nature Communications, represents a significant step toward more effective inflammation management.
Reference:
Gao, Cheng, et al. “Targeted therapies of inflammatory diseases with intracellularly gelated macrophages in mice and rats.” Nature communications 15.1 (2024): 328.
