Editor: Tiffany
A newly developed nanocomposite combining amine-functionalized metal-organic frameworks with magnetic nanoparticles enables precise co-delivery of doxorubicin and pCRISPR to cancer cells, improving therapeutic efficacy and reducing toxicity.
Key Highlights
- Research Question:
Can a nanocomposite system be engineered to co-deliver doxorubicin (DOX) and pCRISPR to cancer cells, enhancing targeting precision and therapeutic outcomes? - Research Difficulties:
Challenges include achieving high drug loading efficiency, ensuring effective cellular uptake, optimizing gene transfection, and mimicking the cellular microenvironment for accurate testing. - Key Findings:
The (NH₂)-MIL-125(Ti)@C1 nanocomposite achieved a drug loading efficiency of 75.4%, a 38.3% increase in cellular uptake, and a 38.3% transfection efficiency in A549 cells, with enhanced gene delivery when paired with a cell-imprinted substrate. - Innovative Aspects:
The study integrates amine-functionalized (NH₂)-MIL-125(Ti), a copolymer, and MnFe₂O₄ nanoparticles into a single platform for dual delivery, utilizing a cell-imprinted substrate to simulate the tumor microenvironment. - Importance of the Study:
This approach offers a potential solution to the limitations of conventional chemotherapy by improving drug and gene delivery specificity, reducing systemic toxicity, and providing a model for personalized cancer therapies.
Cancer and Its Treatment Challenges
Hepatocellular carcinoma (HCC) stands as the predominant histological subtype of liver cancer, constituting approximately 90% of all liver cancer cases globally. It ranks as the third leading cause of cancer-related mortality worldwide, with both morbidity and mortality rates on a steady rise. The clinical presentation of HCC often includes symptoms such as abdominal pain, unintended weight loss, jaundice, and persistent fatigue. However, a significant challenge in managing HCC lies in its asymptomatic nature during early stages, with many patients remaining undiagnosed until the disease progresses to advanced, less treatable phases.
Current therapeutic strategies for HCC encompass a range of interventions, including surgical resection, liver transplantation, transcatheter arterial chemoembolization (TACE), and systemic chemotherapy. Despite their application, these treatments are fraught with limitations. Surgical interventions, such as resection and transplantation, are frequently compromised by high rates of tumor recurrence and perioperative mortality. TACE, while effective in localized tumor control, often fails to address systemic disease progression. Chemotherapy, a mainstay for advanced HCC, suffers from low response rates, severe adverse effects, and only marginal improvements in patient survival. These shortcomings highlight an urgent and unmet need for innovative, effective, and safer treatment modalities for HCC.
Goals and Scope of the Nanocomposite Study
A pioneering study, published in the Journal of Nanobiotechnology in 2024, aimed to address these therapeutic gaps by developing a natural, safe, and efficacious nanomedicine for the oral treatment of HCC. Led by researchers Qiang Gao, Nanxi Chen, Baoyi Li, Menghang Zu, Ya Ma, Haiting Xu, Zhenhua Zhu, Rui L. Reis, Subhas C. Kundu, and Bo Xiao from various prestigious institutions, the investigation focused on harnessing lipid nanoparticles (LNPs) extracted from the leaves of Morus nigra L.—commonly known as black mulberry. The primary objectives were to extract and purify these LNPs, characterize their physicochemical and biological properties, and evaluate their potential as a targeted, orally administered therapy for HCC. This approach sought to overcome the limitations of conventional treatments by leveraging natural compounds to achieve both efficacy and safety.
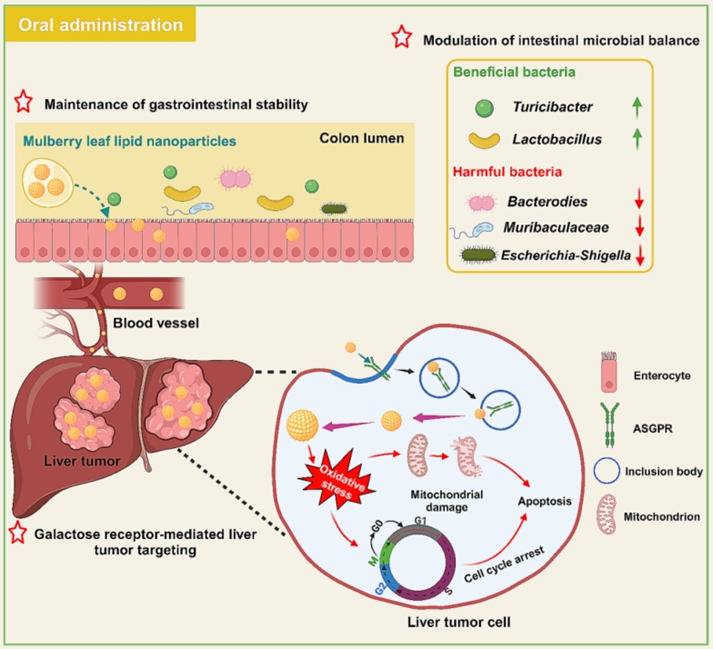
Figure 1. Graphic abstract
Experimental Design and Key Outcomes
The research encompassed a series of methodical steps to evaluate MLNPs:
- Extraction and Purification: MLNPs were isolated from Morus nigra L. leaves using mechanical disruption and purified via centrifugation techniques.
- Characterization: Comprehensive analysis included assessing size, charge, morphology, lipid composition, and bioactive molecule content.
- In Vitro Studies: Experiments focused on cytotoxicity, cellular uptake, and mechanistic insights into MLNPs’ anti-tumor effects.
- In Vivo Studies: Investigations in a murine HCC model assessed biodistribution, therapeutic efficacy, and safety profile of oral MLNPs.
Key Experiment 1: In Vitro Anti-Tumor Activity
- Procedure: HepG2 and Hepa1-6 cells were treated with MLNPs at various concentrations for 24 and 48 hours, followed by viability assessment using the MTT assay.
- Result: IC50 values were 48.6 μg/mL for HepG2 and 74.5 μg/mL for Hepa1-6 at 24 hours, decreasing to 12.0 μg/mL and 17.0 μg/mL at 48 hours, respectively.
- Finding: MLNPs exhibit potent, time-dependent cytotoxicity against liver cancer cells, suggesting their viability as an anti-HCC agent.
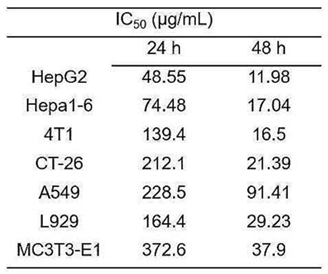
Table 1. IC50 values of MLNPs against various cell lines at 24 and 48 hours.
Key Experiment 2: In Vivo Therapeutic Efficacy
- Procedure: HCC was induced in mice, which were then orally administered MLNPs at 2.5 or 5 mg/kg every three days for five doses. Post-treatment, various biological samples were analyzed.
- Result: Oral MLNPs significantly inhibited tumor growth, improved survival rates, and positively modulated intestinal microbiota with negligible adverse effects.
- Finding: MLNPs demonstrate excellent therapeutic efficacy and safety in a murine HCC model, supporting their potential as an oral nanomedicine for liver cancer treatment.
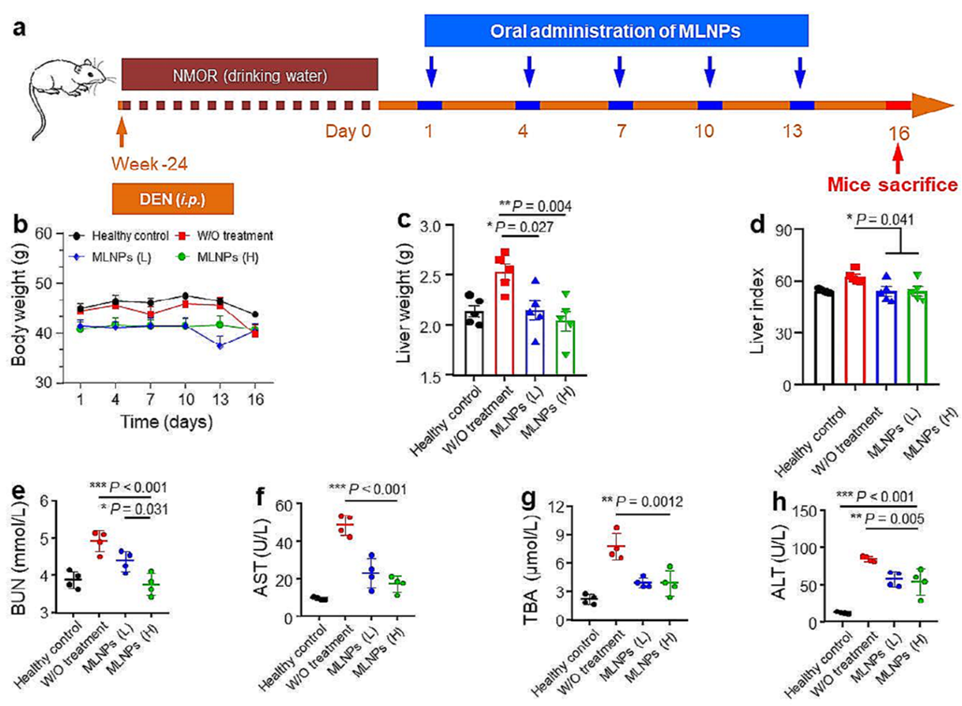
Figure 2. (a) Schematic diagram of the establishment process of orthotopic liver cancer mouse model and treatment process. (b) The body weight, (c) liver weight, and (d) organ index of orthotopic liver cancer mice with the treatment of MLNPs. The amounts of (e) BUN, (f) AST, (g) TBA, and (h) ALT in the serum.
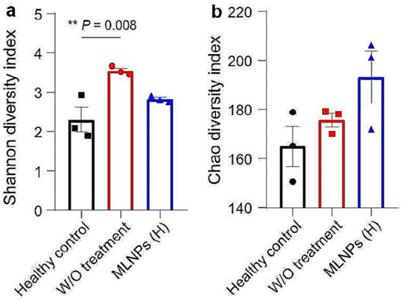
Figure 3. Evaluation of remodeling effects of MLNPs on the intestinal microbiota. α-Diversities were presented by box plots of the (a) Shannon index, and (b) Chao index.
Implications of the Nanocomposite Findings
The study demonstrates that the (NH₂)-MIL-125(Ti)@C1 nanocomposite effectively co-delivers DOX and pCRISPR, with amine functionalization enhancing drug loading, cellular uptake, and transfection efficiency. The use of a cell-imprinted substrate further optimized delivery by simulating the tumor microenvironment. These findings suggest a viable strategy to overcome chemotherapy’s limitations, offering improved specificity and reduced toxicity. The approach provides a foundation for developing targeted combination therapies, potentially advancing personalized cancer treatment.
Reference:
Gao, Qiang, et al. “Natural lipid nanoparticles extracted from Morus nigra L. leaves for targeted treatment of hepatocellular carcinoma via the oral route.” Journal of nanobiotechnology 22.1 (2024): 4.
