Editor: Nina
Scientists enhance the oral bioavailability and brain biodistribution of perillyl alcohol by developing a nanostructured lipid carrier system, aiming to improve therapeutic outcomes for glioblastoma treatment.
Key Preview
- Research Question: The study investigates how to enhance the oral bioavailability and brain biodistribution of perillyl alcohol (POH), a potential antitumor agent for brain tumors, which faces limitations due to poor absorption and distribution.
- Research Design and Strategy: Researchers developed nanostructured lipid carriers (NLCs) as a novel delivery system for POH, employing hot homogenization to create a stable formulation that improves drug delivery and therapeutic efficacy.
- Method: NLCs were characterized for size, morphology, encapsulation efficiency, and drug release profiles. In vivo pharmacokinetic studies were conducted in rats to compare the bioavailability of POH encapsulated in NLCs versus free POH.
- Key Results: The study found that NLCs increased the oral bioavailability of POH two-fold and significantly enhanced its concentration in brain tissue compared to free POH. Encapsulation efficiency was remarkably high at 99.68%.
- Significance of the Research: This research provides a promising approach for improving the delivery of POH to brain tumors, addressing critical pharmacokinetic challenges and potentially enhancing therapeutic outcomes for glioblastoma patients.
Introduction
Glioblastoma multiforme (GBM) is a highly aggressive and malignant tumor of the central nervous system, characterized by rapid growth and a tendency to infiltrate surrounding brain tissue. It is classified as a grade IV astrocytoma by the World Health Organization, and despite advances in treatment, the prognosis for patients diagnosed with GBM remains poor, with a median survival of approximately 15 months. The standard treatment regimen typically includes surgical resection, followed by adjuvant radiotherapy and chemotherapy, most commonly employing temozolomide. While these approaches can temporarily stabilize the disease, they often fail to eliminate cancerous cells completely due to the tumor’s heterogeneous nature and its ability to develop resistance to therapeutic agents.
One common strategy in traditional treatments is the systemic administration of chemotherapeutic agents, which aims to ensure that the drugs reach the tumor through the bloodstream. However, this approach presents significant challenges, primarily due to the blood-brain barrier (BBB), a selective permeability barrier that protects the brain from harmful substances but also limits the effective delivery of many pharmacological agents. As a result, high doses of chemotherapy are often required to achieve therapeutic concentrations in the brain, leading to increased systemic exposure and a higher incidence of adverse side effects, including gastrointestinal toxicity and myelosuppression. Moreover, the extensive first-pass metabolism of drugs like perillyl alcohol (POH), a promising antitumor agent, further complicates their bioavailability and therapeutic efficacy.
To overcome these challenges, innovative drug delivery strategies are being explored, particularly those utilizing nanotechnology. Nanostructured lipid carriers (NLCs) represent a novel approach that enhances the solubility, stability, and bioavailability of poorly soluble drugs. By encapsulating therapeutic agents within lipid-based nanoparticles, NLCs can facilitate targeted delivery to the brain, bypassing the limitations imposed by the BBB. This system not only aims to improve the bioavailability of drugs such as POH but also enhances their therapeutic concentration at the tumor site while minimizing exposure to healthy tissues. The development of NLCs as a drug delivery strategy holds promise for improving treatment outcomes in patients with glioblastoma, addressing critical pharmacokinetic challenges while potentially reducing adverse side effects associated with conventional therapies.
Research Team and Aim
The research was conducted by a collaborative team from the Laboratory of Nanostructured Formulations at Universidade Estadual do Centro-Oeste, Brazil. The study was led by Dr. Rubiana Mara Mainardes, a prominent researcher in the field of drug delivery systems. The research was carried out in 2023 and culminated in the publication of the paper titled “Enhancing Oral Bioavailability and Brain Biodistribution of Perillyl Alcohol Using Nanostructured Lipid Carriers” in the journal Pharmaceuticals.
The primary aim of the research, as articulated by Dr. Mainardes, was to “develop a novel nanostructured lipid carrier (NLC) formulation that could improve the pharmacokinetic properties of perillyl alcohol (POH), thereby enhancing its therapeutic efficacy against glioblastoma.” This ambitious objective sought to address the significant challenges associated with the systemic delivery of chemotherapeutic agents to the brain, particularly in the context of GBM treatment.
Experimental Process
Experiment 1: Preparation of Nanostructured Lipid Carriers (NLCs)
Primary Technique:
The primary technique employed in this study was the hot homogenization method, which is effective for synthesizing nanostructured lipid carriers (NLCs) incorporating perillyl alcohol (POH).
Key Steps:
- Preparation of the Oily Phase: The oily phase was prepared by mixing 700 µL of perillyl alcohol (POH) with 1.3 g of the solid lipid Gelucire® 43/01 and heating the mixture to 53 °C for 10 minutes.
- Preparation of the Aqueous Phase: Separately, an aqueous solution containing 1% polysorbate 80 and 1% soy lecithin was also heated to 53 °C.
- Homogenization: The heated oily phase was added to the aqueous phase and homogenized using an Ultra Turrax homogenizer at 33,000 RPM for 2 minutes.
- Sonication: The resulting pre-emulsion was subjected to sonication using an ultrasonic sonicator for two cycles of 1 minute each at a power of 90 Watts.
- Cooling: The mixture was cooled to 2–8 °C for 5 minutes to facilitate the formation of NLCs.
- Ultracentrifugation: The NLCs were then subjected to ultracentrifugation at 25,240× g for 20 minutes, and the precipitate was redispersed in ultrapure water.
Data Collection and Analysis:
The mean particle size and polydispersity index (PDI) of the NLCs were measured using dynamic light scattering (BIC 90 plus). The morphology was examined using scanning electron microscopy (SEM), and the encapsulation efficiency was determined by quantifying the amount of POH present in the supernatant after ultracentrifugation through high-performance liquid chromatography (HPLC).
Figure 1.SEM images of POH-loaded NLCs at different magniffcation (A) 5.22 Kx and (B) 32.5 Kx
Results:
The NLCs exhibited a mean particle size of 288 ± 23 nm with a PDI of 0.143, indicating a relatively uniform particle size distribution. The encapsulation efficiency reached 99.68%, confirming the effective incorporation of POH.
Novel Aspects:
This experiment highlights the efficacy of utilizing a dual-functionality approach, where POH served as both the drug and the liquid lipid, facilitating high encapsulation efficiency. The hot homogenization method also allowed for the successful creation of stable NLCs, which contrasts with traditional lipid nanoparticle formulations that often struggle with size uniformity and encapsulation rates.
Experiment 2: In Vitro Release Profile
Primary Technique:
The in vitro release profile of POH from the NLCs was evaluated using Franz diffusion cells, which allow for the assessment of drug release kinetics.
Key Steps:
- Preparation of the Receptor Medium: Phosphate-buffered saline (PBS, 50 mM, pH 7.4) was maintained at a temperature of 37 ± 0.5 °C with continuous stirring at 400 RPM.
- Application of NLCs: A 20 µL suspension of POH-loaded NLCs was applied onto a cellulose acetate membrane interposed between the donor and receptor compartments.
- Sampling: At predetermined time intervals (0.5, 1, 2, 4, 8, 12, 24, and 48 hours), 1.0 mL aliquots were collected from the receptor compartment for analysis.
- Analysis of Release Samples: The collected samples were analyzed using HPLC to quantify the amount of POH released over time.
Data Collection and Analysis:
The cumulative release data was plotted against time, and mathematical models (including the Korsmeyer-Peppas and Weibull models) were applied to characterize the release mechanism.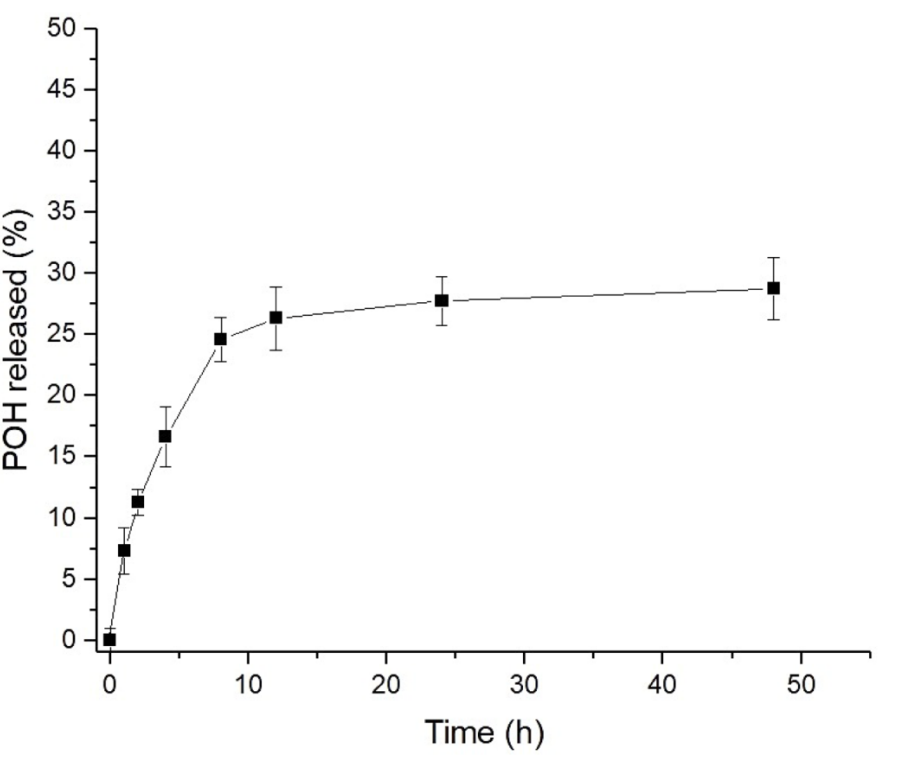
Figure 2. In vitro release of POH from NLCs in PBS pH 7.4, 37 ◦C (n = 3).
Results:
Results:
A biphasic release profile was observed, with approximately 25% of POH released in the first 8 hours and a cumulative release of 29% at 48 hours, indicating controlled release kinetics.
Novel Aspects:
The release profile demonstrated that the NLCs could provide a sustained release of POH, significantly reducing the initial burst effect typical of traditional drug formulations. This controlled release mechanism is advantageous for reducing potential gastrointestinal side effects associated with high-dose oral administration of free POH.
Experiment 3: Pharmacokinetic Studies
Primary Technique:
The pharmacokinetic profile of POH was assessed through an in vivo study using Wistar rats to compare the absorption and distribution of POH in its free form versus as NLCs.
Key Steps:
- Animal Preparation: Adult male Wistar rats were fasted for 12 hours prior to administration. Two groups were formed: one receiving free POH and the other receiving POH-loaded NLCs, with both groups administered a single dose of 500 mg/kg.
- Sample Collection: Blood samples were collected at predetermined time points (0.5, 1, 2, 4, 8, 12, and 24 hours) after administration. The brain and other organs (lungs, kidneys, spleen, liver) were also harvested.
- Analysis of Samples: Plasma and organ homogenates were analyzed using ultra-performance liquid chromatography (UPLC) coupled with mass spectrometry to quantify perillyl acid (PA), a metabolite of POH.
Data Collection and Analysis:
Concentration-time curves were constructed for both plasma and brain samples, and pharmacokinetic parameters such as Cmax, Tmax, AUC0–24h, and half-life (T1/2) were calculated for statistical comparison between groups.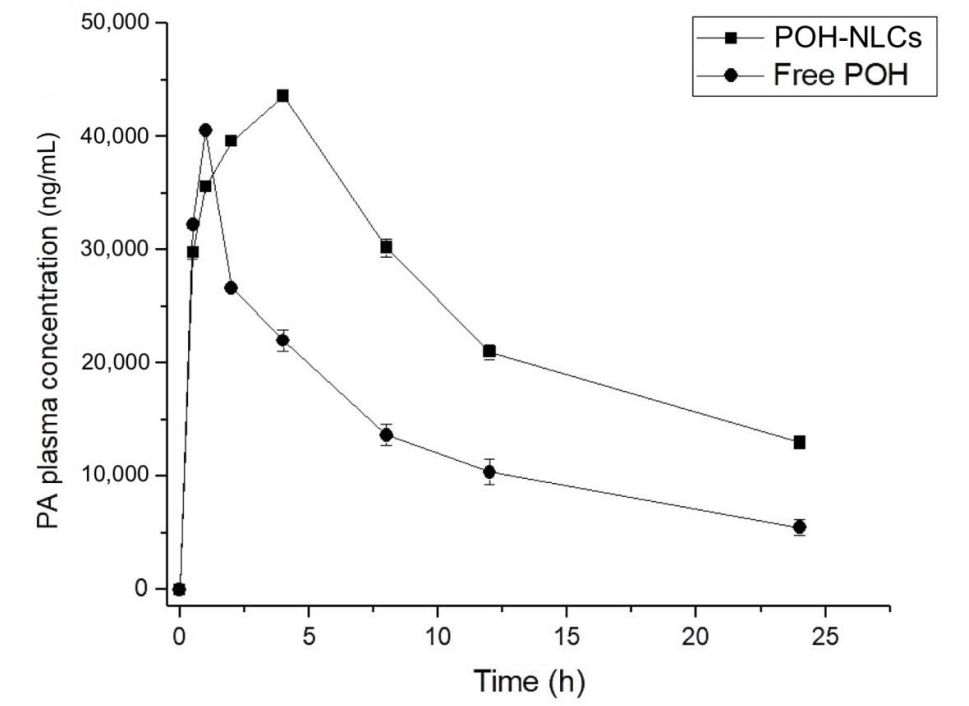
Figure 3. Plasma concentration–time curves of perillyl acid (PA) obtained after single oral administration of 500 mg/kg of POH in rats, using NLCs and in its free form.
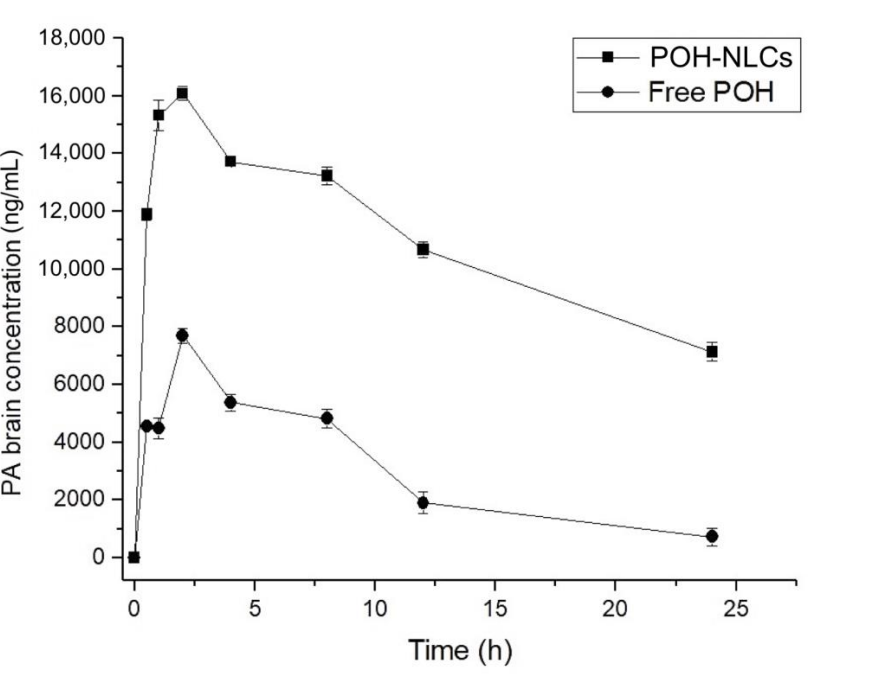
Figure 4. Brain concentration–time curves of perillyl acid (PA) obtained after single oral administration of 500 mg/kg of POH in rats, using NLCs and in its free form.
Results:
The NLCs significantly enhanced the oral bioavailability of POH, with a two-fold increase in AUC0–24h compared to its free form. The maximum brain concentration of PA was also significantly higher in the NLC group, indicating improved brain biodistribution.
Novel Aspects:
The study’s innovative approach using NLCs demonstrated a marked improvement in pharmacokinetics over free POH, highlighting their potential for targeted drug delivery to the brain. This advancement addresses the significant challenge of achieving effective concentrations of therapeutics in brain tissue while minimizing systemic exposure and potential side effects.
Conclusion
The successful development of the nanostructured lipid carrier (NLC) system for enhancing the oral bioavailability and brain biodistribution of perillyl alcohol (POH) was achieved through a meticulously designed experimental process that included the synthesis, characterization, and pharmacokinetic evaluation of the NLCs. By employing the hot homogenization technique, the research team was able to create a stable formulation with high encapsulation efficiency and desirable physicochemical properties, such as a mean particle size suitable for targeted delivery and a low polydispersity index indicating uniformity.
The study highlighted several key findings, including the remarkable two-fold increase in oral bioavailability of POH when encapsulated in NLCs compared to its free form. Additionally, the NLCs significantly enhanced the concentration of POH in brain tissue, indicating improved brain biodistribution and suggesting a promising therapeutic strategy for glioblastoma treatment. Furthermore, the sustained release profile demonstrated that the NLCs could minimize the gastrointestinal side effects typically associated with high-dose oral administration of free POH.
Overall, this research represents a significant advancement in the field of drug delivery systems, offering a novel approach to overcoming the pharmacokinetic challenges faced in the treatment of brain tumors and paving the way for improved therapeutic outcomes in patients with glioblastoma multiforme.
Reference
Peczek, Samila Horst, et al. “Enhancing Oral Bioavailability and Brain Biodistribution of Perillyl Alcohol Using Nanostructured Lipid Carriers.” Pharmaceuticals, vol. 16, no. 8, 2023, Article 1055. MDPI,
https://doi.org/10.3390/ph16081055.
