Editor: Tiffany
A study demonstrates that optimized albumin nanoparticles significantly improve the bioavailability and anti-cancer efficacy of silymarin in treating hepatocellular carcinoma, utilizing a Quality by Design framework for formulation.
Key Highlights
- Research Question:
Can the formulation and optimization of albumin nanoparticles loaded with silymarin improve its bioavailability and effectiveness against hepatocellular carcinoma? - Research Difficulties:
The study faced challenges regarding the low bioavailability of silymarin and the complex formulation process of albumin nanoparticles influenced by numerous variables. - Key Findings:
The optimized nanoparticles demonstrated a particle size of 135 nm and an entrapment efficiency of 88%, showing enhanced anti-proliferative activity against the HepG2 liver cancer cell line compared to free silymarin. - Innovative Aspects:
The use of a Quality by Design (QbD) approach allowed for a comprehensive understanding of product and process parameters to optimize the nanoparticle formulation. - Importance of the Study:
This research provides a promising new strategy for targeted cancer therapy, potentially improving treatment outcomes for patients with hepatocellular carcinoma.
Challenges in Hepatocellular Carcinoma Therapy
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and a leading cause of cancer-related deaths worldwide. The incidence of HCC has been rising, particularly in regions with high rates of viral hepatitis and liver cirrhosis. Symptoms often include abdominal pain, weight loss, and jaundice, which can complicate early diagnosis and lead to advanced disease at the time of detection. Current therapeutic options for HCC include surgical resection, liver transplantation, and various forms of chemotherapy. However, traditional chemotherapy is often limited by systemic toxicity and inadequate efficacy against tumor cells. Consequently, innovative drug delivery methods are urgently required to enhance treatment efficacy while minimizing side effects. Nano-drug delivery systems, particularly those utilizing biocompatible materials like albumin, have shown promise in improving drug bioavailability and targeting tumor tissues more effectively, offering a potential solution for more successful cancer therapies.
Objectives and Approach of the Study
The primary aim of this research was to formulate and optimize albumin nanoparticles loaded with silymarin, a natural compound known for its hepatoprotective and potential anticancer properties, to improve its bioavailability and therapeutic efficacy against hepatocellular carcinoma. The study specifically sought to employ a Quality by Design (QbD) approach to systematically understand and control the formulation process. Key objectives included identifying the critical formulation parameters that influence the size and entrapment efficiency of the nanoparticles, conducting a comprehensive risk assessment to pinpoint the most significant factors affecting product quality, and utilizing a D-optimal design to optimize the formulation for enhanced delivery of silymarin to liver cancer cells. Ultimately, the research aimed to demonstrate the effectiveness of the optimized albumin nanoparticles in inhibiting the growth of hepatocellular carcinoma cells in vitro, providing a foundation for future therapeutic applications.
Experimental Design and Key Findings
Experimental Process Outline
- Conduct a thorough risk assessment for the formulation of albumin nanoparticles.
- Define the Quality Target Product Profile (QTPP) using the Quality by Design (QbD) approach.
- Prepare silymarin-loaded albumin nanoparticles using the desolvation technique.
- Optimize formulation parameters using a D-optimal design.
- Assess particle size, polydispersity index (PDI), and zeta potential using photon correlation spectroscopy.
- Perform transmission electron microscopy (TEM) for morphological analysis of nanoparticles.
- Measure entrapment efficiency (EE%) and percentage yield of nanoparticles.
- Evaluate in vitro release of silymarin from nanoparticles using the dialysis bag method.
- Conduct cytotoxicity assays on HepG2 cells using the sulforhodamine B (SRB) assay.
- Analyze cell cycle distribution using flow cytometry.
- Perform molecular docking studies to explore interactions between silymarin and bovine serum albumin.
Key Experiments
Experiment 1: Formulation and Optimization of Albumin Nanoparticles
Procedure:
- Utilize the desolvation technique to prepare the albumin nanoparticles.
- Dissolve bovine serum albumin in distilled water and adjust pH to 8 or 9.
- Dissolve silymarin in ethanol as the desolvating agent and add it to the albumin solution.
- Induce cross-linking by adding glutaraldehyde and stirring for a specified time.
- Purify the nanoparticles through centrifugation and re-dispersion.
Result:
- The optimized formulation yielded nanoparticles with a particle size of 135 nm, a PDI of 0.09, and an EE% of 88%.
Finding:
- The successful formulation of silymarin-loaded albumin nanoparticles demonstrates the effectiveness of the QbD approach in optimizing the characteristics crucial for drug delivery.
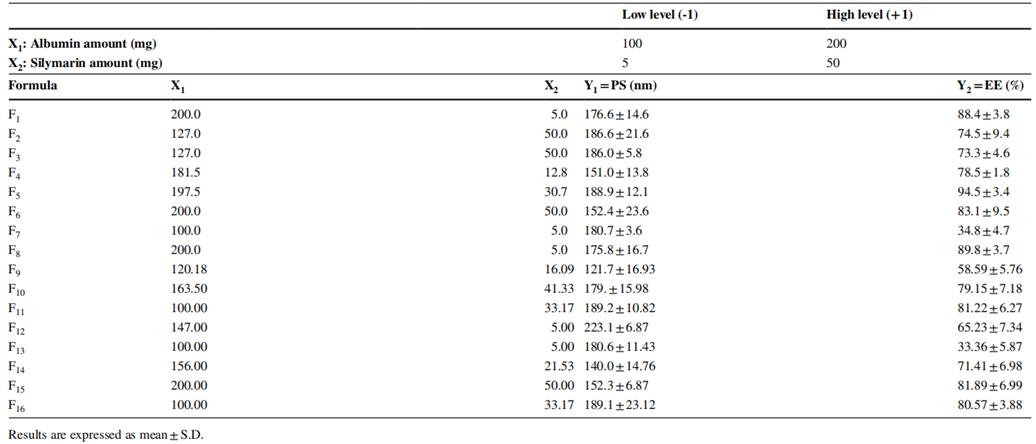
Table 1. D-Optimal design study with results from the optimization step.
Experiment 2: In Vitro Release Study
Procedure:
- Use dialysis bags to assess the in vitro release of silymarin from the albumin nanoparticles and compare with the free drug.
- Place equivalent amounts of the optimized formula and free silymarin in the dialysis bags immersed in phosphate-buffered saline (PBS) at 37 °C.
- Withdraw samples at predetermined time intervals and measure absorbance spectrophotometrically.
Result:
- The release profile showed an initial burst release of approximately 45% within 4 hours, followed by a slower, sustained release over 48 hours for the optimized formula. In contrast, the free silymarin was completely released within 3 hours.
Finding:
- The biphasic release pattern indicates that the albumin nanoparticles can provide sustained drug release, which is advantageous for minimizing side effects while maximizing therapeutic efficacy in targeted cancer treatment.
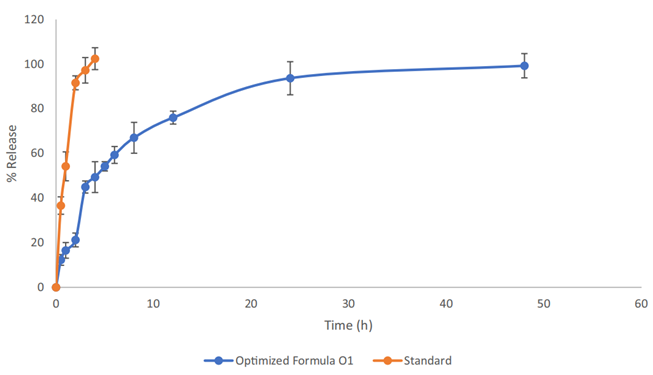
Figure 1. Release pattern of silymarin from the optimized formula and standard silymarin.
Experiment 3: Cytotoxicity Assay on HepG2 Cells
Procedure:
- Treat HepG2 cells with varying concentrations of both free silymarin and the optimized albumin nanoparticles for 72 hours.
- Assess cell viability using the sulforhodamine B (SRB) assay by measuring absorbance at 540 nm post-treatment.
- Calculate the IC50 values for both formulations to compare their effectiveness.
Result:
- The IC50 for the optimized formula was found to be 84 μM, while the free silymarin exhibited an IC50 of 140 μM, indicating a greater potency of the nanoparticles.
Finding:
- The enhanced cytotoxicity of silymarin-loaded albumin nanoparticles against HepG2 cells suggests that the optimized formulation significantly improves the therapeutic potential of silymarin in treating hepatocellular carcinoma.
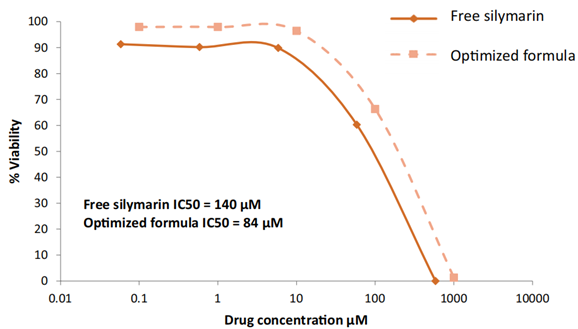
Figure 2. Effects of free silymarin and optimized silymarin albumin nanoparticles on the proliferation of HepG2 cells.
Conclusions and Future Directions
This study successfully developed and optimized albumin nanoparticles loaded with silymarin, utilizing a Quality by Design (QbD) approach to enhance the bioavailability and therapeutic efficacy of this natural compound against hepatocellular carcinoma. The innovative aspect of this research lies in its systematic identification and optimization of critical formulation parameters, which resulted in nanoparticles with a particle size of 135 nm, an entrapment efficiency of 88%, and a favorable zeta potential of −12.5 mV. Notably, the in vitro release profile exhibited a biphasic pattern, characterized by an initial burst release followed by sustained release over 48 hours, indicating the potential for targeted delivery and minimized side effects. Cytotoxicity assays revealed that the optimized silymarin-loaded albumin nanoparticles demonstrated significantly greater antiproliferative activity against HepG2 cells compared to free silymarin, with an IC50 value of 84 μM versus 140 μM for the free drug. This finding underscores the enhanced effectiveness of the nanoparticle formulation in inhibiting cancer cell growth.
In conclusion, the study highlights the successful application of the QbD framework in the formulation of silymarin albumin nanoparticles, achieving a comprehensive understanding of product and process parameters. The optimized nanoparticles not only demonstrate promising results in vitro but also hold potential for future clinical applications in targeted therapy for hepatocellular carcinoma.
Reference:
Dawoud, Marwa HS, et al. “A quality by design paradigm for albumin-based nanoparticles: formulation optimization and enhancement of the antitumor activity.” Journal of pharmaceutical innovation 18.3 (2023): 1395-1414.
