Editor: Nina
Scientists develop dual-peptide functionalized nanoparticles to enhance blood-brain barrier penetration and cytotoxicity of Palbociclib in U87-MG glioblastoma cells for improved therapeutic outcomes.
Key Preview
- Research Question: The study investigates how to enhance the delivery and cytotoxicity of Palbociclib (PBC) in glioblastoma cells by utilizing dual peptide-functionalized nanoparticles to improve blood-brain barrier (BBB) penetration.
- Research Design and Strategy: Researchers designed and synthesized nanoparticles (NPs) functionalized with specific peptides (T7 and R9) to enhance both BBB penetration and targeted cytotoxic effects on U87-MG glioblastoma cells.
- Method: The study involved the preparation of PBC-loaded nanoparticles, characterization of their size and zeta potential, and evaluation of their transport across a BBB model, followed by in vitro cytotoxicity assessments.
- Key Results: The dual-peptide modified nanoparticles showed superior transport efficiency across the BBB (92.7% compared to 41.8% for free PBC) and significantly improved apoptosis in U87-MG cells (2.3-6.5 times greater than free PBC).
- Significance of the Research: This research provides an innovative approach to overcoming the challenges of drug delivery to the brain, potentially improving therapeutic outcomes for glioblastoma patients through enhanced delivery of CDK4/6 inhibitors like PBC.
Introduction
Glioblastoma is one of the most aggressive and devastating forms of brain cancer, characterized by its rapid growth, infiltrative nature, and resistance to conventional therapies. This malignancy arises from glial cells and poses significant challenges for treatment due to its complex tumor microenvironment and the presence of the blood-brain barrier (BBB), which effectively limits the penetration of therapeutic agents into the brain. Patients diagnosed with glioblastoma typically face a poor prognosis, with a median survival time of approximately one year and only 5% surviving beyond five years. The aggressive behavior of this cancer underscores the urgent need for effective therapeutic strategies.
Traditional treatment for glioblastoma often involves a combination of surgical resection, radiation therapy, and systemic chemotherapy. The most common chemotherapeutic agents, such as temozolomide, are administered systemically to target tumor cells. However, this approach is fraught with limitations. The BBB, a selective permeability barrier formed by tightly packed endothelial cells, restricts the entry of many therapeutic compounds, leading to suboptimal drug delivery to the tumor site. Additionally, the cytotoxic effects of chemotherapy on healthy brain tissue can result in significant side effects, further complicating treatment. Consequently, the limited effectiveness of conventional treatments contributes to high rates of tumor recurrence and poor overall survival rates.
The challenges associated with traditional drug delivery methods highlight the need for innovative strategies that can effectively penetrate the BBB and specifically target glioblastoma cells. One promising approach involves the use of nanoparticle-based delivery systems that can encapsulate chemotherapeutic agents and facilitate their transport across the BBB. By engineering nanoparticles with specific targeting ligands, researchers can enhance the selective uptake of drugs by tumor cells while minimizing exposure to healthy tissues. This innovative drug delivery strategy not only aims to improve the bioavailability of therapeutic agents but also seeks to enhance their efficacy in inducing apoptosis in cancer cells. The development of dual peptide-functionalized nanoparticles—utilizing peptides that target specific receptors on glioma cells—represents a significant advancement in overcoming the barriers associated with conventional drug delivery methods and offers hope for improved outcomes in glioblastoma treatment.
Research Team and Aim
The research team behind this study consists of Yu-Chen Lo and Wen-Jen Lin, both affiliated with the School of Pharmacy at National Taiwan University. The research was conducted over a timeframe culminating in its publication on October 6, 2023. The study was titled “Improve BBB Penetration and Cytotoxicity of Palbociclib in U87-MG Glioblastoma Cells Delivered by Dual Peptide Functionalized Nanoparticles” and was published in the journal Pharmaceutics.
The primary aim of the research, as articulated by lead researcher Wen-Jen Lin, is to develop a nanoparticle delivery system capable of enhancing the transport of Palbociclib (PBC) across the blood-brain barrier (BBB) and its subsequent cytotoxic effects on glioblastoma cells. This innovative approach seeks to address the significant challenges associated with traditional drug delivery methods and improve therapeutic outcomes for patients suffering from glioblastoma.
Experimental Process
Primary Technique: Nanoparticle Synthesis and Characterization
The main method used in this study involved the synthesis and characterization of dual peptide-functionalized nanoparticles (NPs) to enhance the delivery of Palbociclib (PBC) in glioblastoma cells. This technique combines polymeric nanoparticle formulation with peptide conjugation, leveraging the unique properties of the peptides to facilitate targeted delivery across the blood-brain barrier (BBB).
Experiment 1: Synthesis of Peptide-Conjugated PLGA-PEG Copolymers
Key Steps:
- Poly(D,L-lactide-co-glycolide) (PLGA) was pre-activated to PLGA-NHS.
- PLGA-NHS was then reacted with different chain lengths of polyethylene glycol (PEG) (5k and 2k) at a molar ratio of 1:1.5 in the presence of N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) at room temperature for 24 hours.
- The T7 and R9 peptides were subsequently conjugated to the PLGA-PEG copolymers.
Data Collection and Analysis:
The synthesized copolymers were characterized using size exclusion chromatography (SEC) to determine their molecular weight and polydispersity index (PDI). The critical micelle concentration (CMC) was also assessed using pyrene as a fluorescence probe.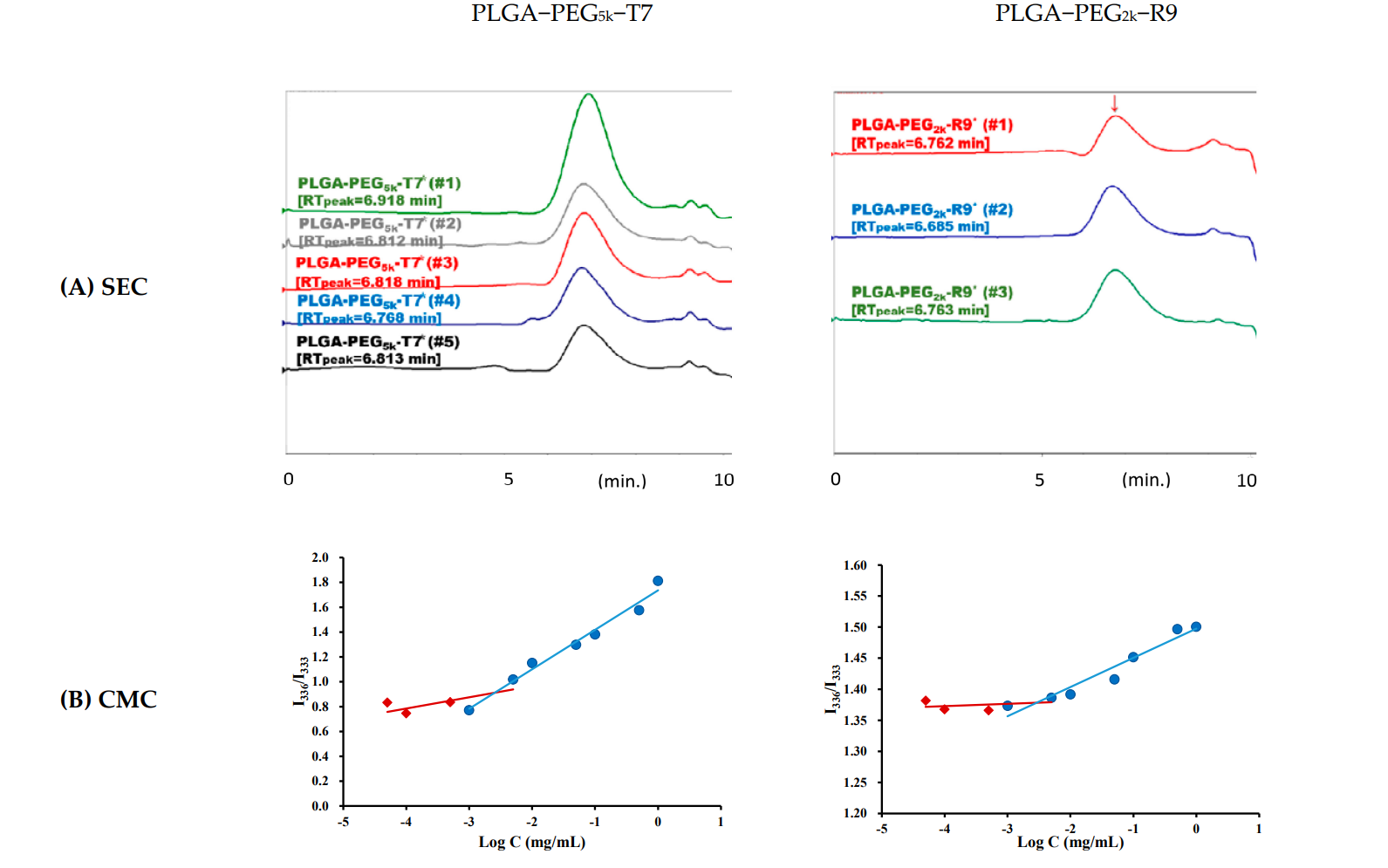
Figure 1. (A) SEC chromatograms of PLGA-PEG5k-T7 (n = 5) and PLGA-PEG2k-R9 (n = 3) including the retention time. (B) CMC plots of PLGA-PEG5k-T7 and PLGA-PEG2k-R9 using pyrene as a fluorescence probe. The emission wavelength was 390 nm, and the excitation fluorescence was recorded at 336 nm and 333 nm. The concentration at the intersection of the steep and the horizontal parts of the curve corresponds to the CMC value.
Result:
The synthesized PLGA-PEG5k-T7 and PLGA-PEG2k-R9 copolymers exhibited molecular weights of 49,600 g/mol and 56,000 g/mol, respectively, with a CMC of 2.53 × 10^-3 mg/mL for PLGA-PEG5k-T7.
Novel Aspects:
This experiment introduced a method for functionalizing PLGA-PEG copolymers with specific peptides, enhancing their potential for targeted drug delivery compared to traditional polymeric nanoparticles that lack such targeted functionalities.
Experiment 2: Preparation of PBC-Loaded Nanoparticles (PBC@NPs)
Key Steps:
- The PBC-loaded nanoparticles were prepared using a single solvent evaporation method, where the polymer and PBC were dissolved in dichloromethane as the oil phase.
- Polyvinyl alcohol (PVA) was used as the water phase, and both phases were mixed under sonication in an ice bath.
- The organic solvent was removed using a rotary evaporator, and the resulting nanoparticles were collected through centrifugation.
Data Collection and Analysis:
Particle size, zeta potential, and encapsulation efficiency (EE) were measured using a zetasizer and HPLC. The EE was calculated based on the amount of PBC recovered from the nanoparticles compared to the initial amount used.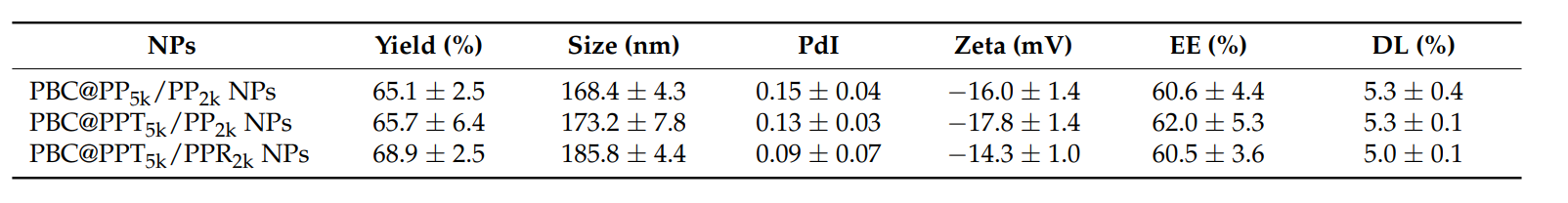
Table 1. Characteristics of PBC@NPs. (n = 3, mean ± SD)
Result:
The resulting PBC@NPs had a particle size range of 168.4 nm to 185.8 nm and an EE of approximately 60% across various formulations.
Novel Aspects:
This method provided a simple and efficient way to encapsulate PBC in peptide-functionalized nanoparticles, improving drug loading and stability compared to conventional methods that typically have lower encapsulation efficiencies.
Experiment 3: In Vitro Release Studies
Key Steps:
- The release profile of PBC from the nanoparticles was examined in two different pH conditions (physiological pH 7.4 and acidic pH 5.5).
- PBC@NPs were suspended in the release media, placed in dialysis bags, and incubated in a shaking water bath at 37 °C.
- At specific time intervals, samples of the release medium were collected and analyzed for PBC concentration using HPLC.
Data Collection and Analysis:
The cumulative release of PBC was plotted against time, and the release kinetics were evaluated using a suitable model to determine the release mechanism.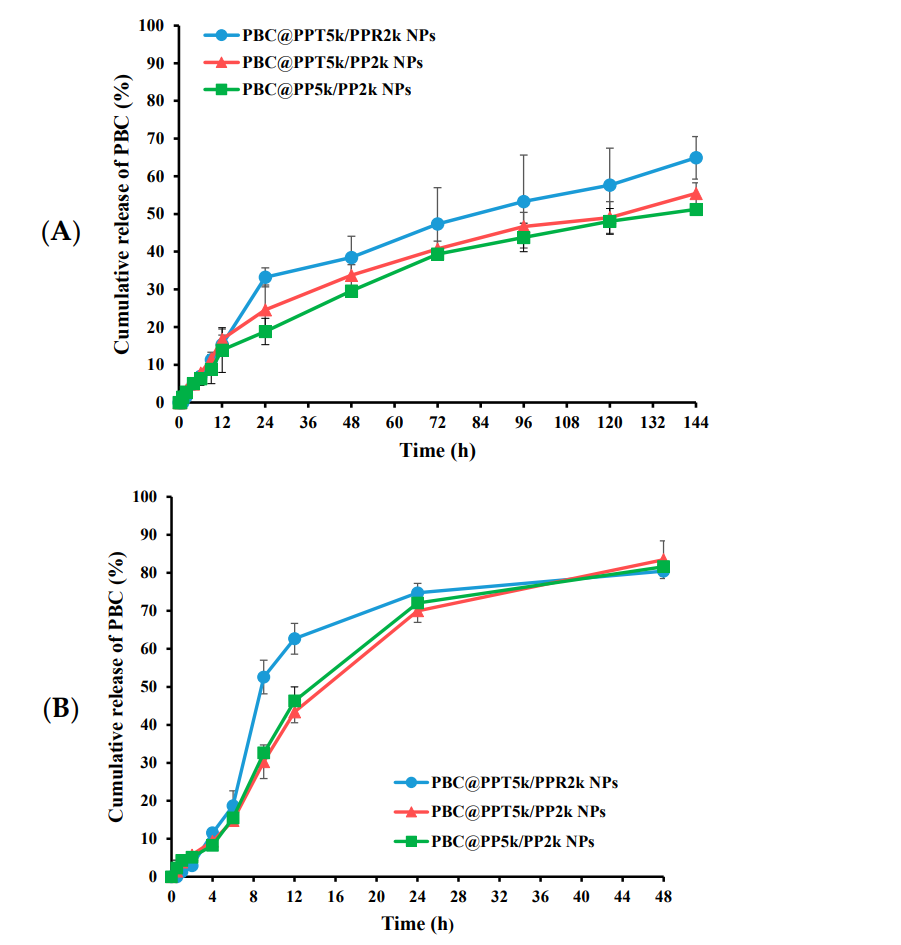
Figure 2. cumulative release of PBC from PBC@NPs in (A) pH 7.4 and (B) pH 5.5 release media. (n = 3, mean ± SD).
Result:
The study found that PBC was released at a significantly higher rate in the acidic environment (pH 5.5) compared to physiological conditions, with approximately 80% of PBC released in 48 hours at pH 5.5.
Novel Aspects:
This pH-dependent release mechanism provides a significant advantage over traditional systems by allowing for targeted release in acidic tumor microenvironments, which enhances therapeutic efficacy.
Experiment 4: Cellular Uptake in a Co-Cultured BBB Model
Key Steps:
- A co-culture of bEnd.3 and U87-MG cells was established in a Transwell® system to mimic the BBB.
- FITC-labeled peptide-free and T7-modified nanoparticles were introduced to the upper chamber at varying concentrations.
- After 4 and 12 hours of incubation, the cells were trypsinized, washed, and analyzed for fluorescence intensity using flow cytometry.
Data Collection and Analysis:
Flow cytometric analysis enabled quantification of the cellular uptake of nanoparticles in both bEnd.3 and U87-MG cells, with mean fluorescence intensity (MFI) used as a measure of uptake.
Result:
The T7-modified nanoparticles showed significantly enhanced uptake in U87-MG cells compared to peptide-free nanoparticles, particularly after 12 hours of incubation.
Novel Aspects:
This experiment demonstrated the effectiveness of T7 peptide in enhancing cellular uptake across the BBB, surpassing the capabilities of traditional nanoparticles that do not utilize targeting ligands, thus showing promise for improved brain tumor therapy.
Experiment 5: Transport of PBC@NPs across bEnd.3 Cell Model
Key Steps:
- The transport efficiency of PBC@NPs was assessed using a bEnd.3 monolayer model.
- PBC solution and various formulations of PBC@NPs were added to the upper chamber of the Transwell® system.
- Samples from the lower chamber were collected at specified intervals to measure the concentration of PBC via HPLC.
Data Collection and Analysis:
Transport efficiency was calculated as the percentage of PBC that crossed the bEnd.3 monolayer compared to the initial concentration added.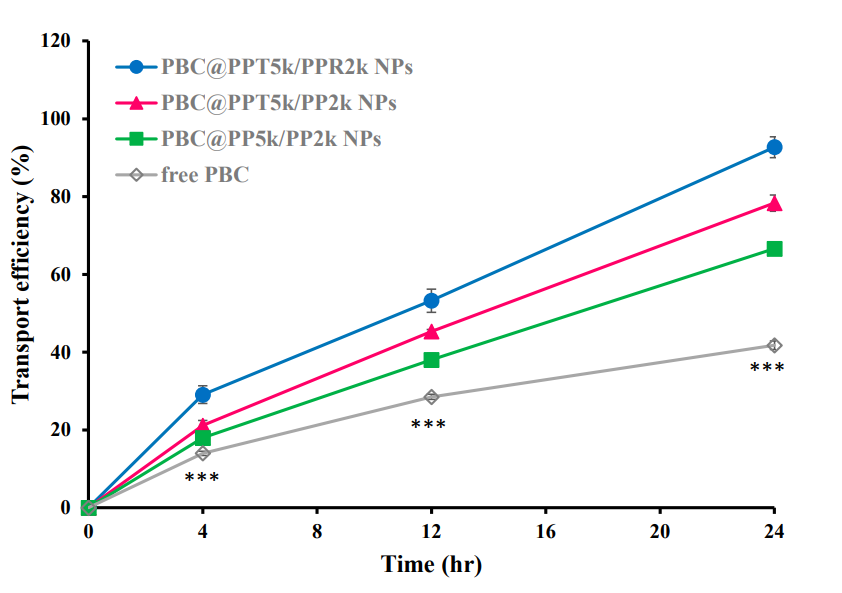
Figure 3. In vitro transport of PBC@NPs (PBC@PP5k/PP2k NPs, PBC@PPT5k/PP2k NPs, and PBC@PPT5k/PPR2k NPs) and PBC solution across the bEnd.3 monolayer. (n = 3, mean ± SD, *** p <0.001:free PBC compared to PBC @NPs).
Result:
The dual-peptide modified PBC@NPs achieved a transport efficiency of 92.7%, significantly higher than free PBC (41.8%).
Novel Aspects:
The ability of dual-peptide modified nanoparticles to significantly enhance drug transport across the BBB represents a major advancement in the design of nanoparticle systems for brain-targeted therapies, providing a more effective approach than traditional methods.
Conclusion
The successful development of the dual-peptide functionalized nanoparticle (NP) system for delivering Palbociclib (PBC) to glioblastoma cells was achieved through a series of meticulously designed experiments that focused on overcoming the challenges of traditional drug delivery methods. By functionalizing poly(lactide-co-glycolide) (PLGA) with specific targeting ligands, namely the T7 peptide and the R9 cell-penetrating peptide, the research team effectively enhanced both the transport of PBC across the blood-brain barrier (BBB) and its cytotoxic effects on U87-MG glioblastoma cells.
The highlights of the study include the significant increase in transport efficiency of PBC@NPs across the BBB, achieving a transport rate of 92.7% with the dual-peptide modified nanoparticles compared to only 41.8% for free PBC. Furthermore, the PBC@NPs demonstrated a remarkable enhancement in inducing apoptosis in U87-MG cells, with a 2.3 to 6.5-fold increase in cytotoxicity relative to free PBC. These findings illustrate the potential of the dual-peptide functionalized NP system not only to improve drug delivery to the brain but also to significantly enhance the therapeutic efficacy of CDK4/6 inhibitors like PBC in the treatment of glioblastoma. This innovative approach represents a promising advancement in the quest for more effective therapies against this challenging malignancy.
Reference
Lo, Yu-Chen, and Wen-Jen Lin. “Improve BBB Penetration and Cytotoxicity of Palbociclib in U87-MG Glioblastoma Cells Delivered by Dual Peptide Functionalized Nanoparticles.” Pharmaceutics, vol. 15, no. 10, 2023, article 2429. MDPI, https://doi.org/10.3390/pharmaceutics15102429.
