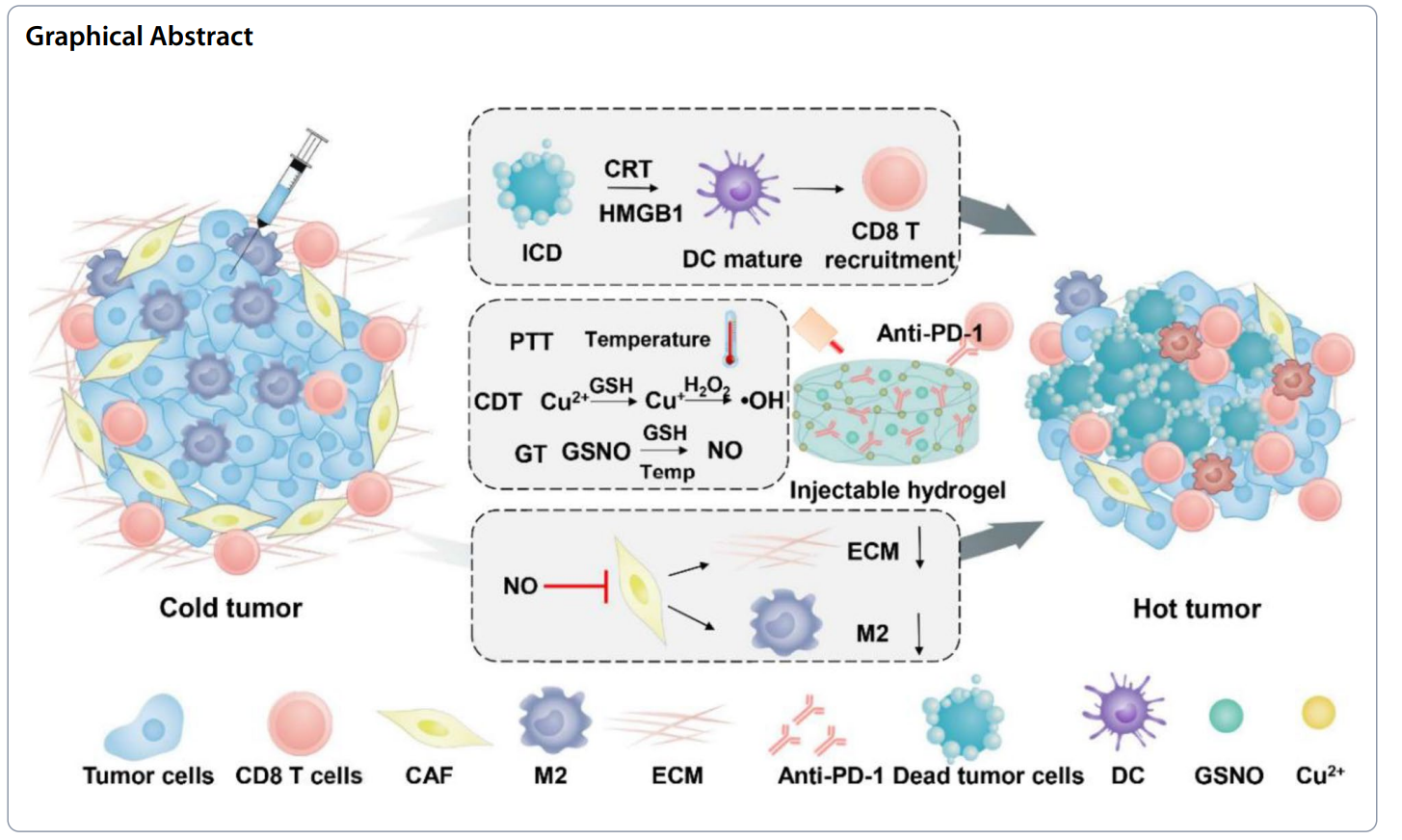
Scientists develop a copper-induced injectable hydrogel that releases nitric oxide to enhance cancer immunotherapy by amplifying immunogenic cell death and regulating the tumor microenvironment through the depletion of cancer-associated fibroblasts.
Key Preview
- Research Question: How can a copper-induced injectable hydrogel, in conjunction with nitric oxide, enhance immunotherapy by promoting immunogenic cell death and modulating the behavior of cancer-associated fibroblasts (CAFs)?
- Research Design and Strategy: The study employed an injectable hydrogel containing copper ions and a nitric oxide donor to explore its effects on cancer cell viability and immune response in both in vitro and in vivo settings.
- Method: The therapeutic efficacy was assessed using 4T1 breast cancer cells and CAFs in vitro, along with 4T1 tumor-bearing mice in vivo. Techniques included flow cytometry, enzyme-linked immunosorbent assay (ELISA), immunofluorescence, and transcriptomic analysis.
- Key Results: The hydrogel demonstrated a persistent photothermal effect, enhancing immunogenic cell death and promoting a robust immune response by depleting CAFs and reducing the proportion of M2-type macrophages.
- Significance of the Research: This innovative approach offers a promising strategy to improve cancer immunotherapy efficacy by reversing the immunosuppressive tumor microenvironment.
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, characterized by the uncontrolled proliferation of abnormal cells that can invade surrounding tissues and metastasize to distant organs. Despite advancements in treatment modalities such as surgery, chemotherapy, and radiation therapy, the complexity of cancer biology often results in limited therapeutic efficacy and significant side effects. Among various treatment strategies, immunotherapy—particularly immune checkpoint blockade (ICB)—has gained recognition for its potential to harness the body’s immune system to target and destroy tumor cells. However, the effectiveness of ICB is frequently hampered by the low immunogenicity of cancer cells and the presence of immunosuppressive components within the tumor microenvironment (TME), such as cancer-associated fibroblasts (CAFs) and regulatory T cells.
Traditional drug delivery systems commonly rely on small-molecule chemotherapeutics or monoclonal antibodies that are administered systemically. While this approach can effectively target rapidly dividing cancer cells, it often lacks specificity, resulting in off-target effects and toxicity to healthy tissues. Furthermore, the heterogeneous nature of tumors can lead to suboptimal drug penetration and uneven distribution, which compromises treatment efficacy and contributes to tumor recurrence and metastasis. The immunosuppressive TME further complicates this scenario, as CAFs and other stromal components can inhibit the infiltration and functionality of immune cells, diminishing the therapeutic impact of immunotherapies.
To address these challenges, innovative drug delivery strategies are being developed to enhance the specificity and efficacy of cancer therapies. One such strategy involves the use of injectable hydrogels that can provide localized drug delivery while simultaneously modulating the TME. These hydrogels can be engineered to encapsulate therapeutic agents such as nitric oxide (NO) donors and immune checkpoint inhibitors, enabling sustained release at the tumor site. By combining photothermal therapy and chemodynamic therapy with immunotherapy, the hydrogel approach aims to amplify immunogenic cell death and reverse the immunosuppressive effects of CAFs. This multifaceted strategy not only improves drug delivery to tumor cells but also enhances immune cell infiltration and activation, paving the way for more effective cancer treatment.
Research Team and Aim
The research team behind this innovative study is composed of experts from Nanjing Tech University, including Shuilin Shen, Zimeng Zhang, Haixiao Huang, Jing Yang, Xinyue Tao, Zhengjie Meng, Hao Ren, and Xueming Li. The lead researcher, Hao Ren, conducted this research during a period of significant advancement in cancer therapy strategies, culminating in the publication of their findings in 2023. The paper is titled “Copper-induced injectable hydrogel with nitric oxide for enhanced immunotherapy by amplifying immunogenic cell death and regulating cancer-associated fibroblasts” and was published in the journal Biomaterials Research.
The aim of the research, as articulated by lead researcher Hao Ren, is to explore a novel therapeutic strategy that utilizes a hydrogel to amplify immunogenic cell death and modulate the behavior of cancer-associated fibroblasts (CAFs), thereby enhancing the efficacy of cancer immunotherapy. The study seeks to provide a comprehensive approach to overcoming the limitations posed by the immunosuppressive tumor microenvironment, ultimately contributing to more effective cancer treatments.
Experimental Process
1. Preparation of the Injectable Hydrogel
Primary Technique: The main method employed in this study was the synthesis of a copper-induced injectable hydrogel via the chelation of mercaptohyaluronic acid (HA-SH) with copper ions (Cu²⁺).
Key Steps:
- Synthesis of HA-SH: HA was dissolved in distilled water, and EDC·HCl and NHS were added to activate the carboxyl groups. After adjusting the pH to 4.5, cystamine dihydrochloride was introduced to introduce thiol groups. The mixture was subsequently dialyzed to purify HA-SH.
- Hydrogel Formation: The synthesized HA-SH was mixed with varying concentrations of CuCl₂ to form a cross-linked hydrogel through coordination between the thiol groups and copper ions.
Data Collection and Analysis: The formation of the hydrogel was confirmed using infrared spectroscopy to detect the presence of thiol groups. The mechanical properties were evaluated via dynamic rheometry.
Result: The resultant hydrogel exhibited a stable three-dimensional network structure, as evidenced by the rheological measurements showing consistent G’ and G’’ values, indicating its potential for sustained release applications.
Novel Aspect: This method of creating a hydrogel using copper ions uniquely combines photothermal effects and chemodynamic therapy into a single delivery system, enhancing therapeutic efficacy compared to traditional nanoparticle systems which often lack such multifunctionality.
2. Evaluation of Photothermal Effects
Primary Technique: The photothermal properties of the hydrogel were assessed using near-infrared (NIR) laser irradiation.
Key Steps:
- Temperature Measurement: Hydrogel samples were placed in a 96-well plate and irradiated with an 808 nm laser at a power density of 0.8 W/cm² for 140 seconds. The temperature was recorded at set intervals using a K-type thermometer.
Data Collection and Analysis: The temperature changes were documented and analyzed to determine the heating efficiency of the hydrogel.
Result: The hydrogel demonstrated a significant temperature increase of approximately 35°C, indicating effective photothermal conversion due to the presence of copper ions.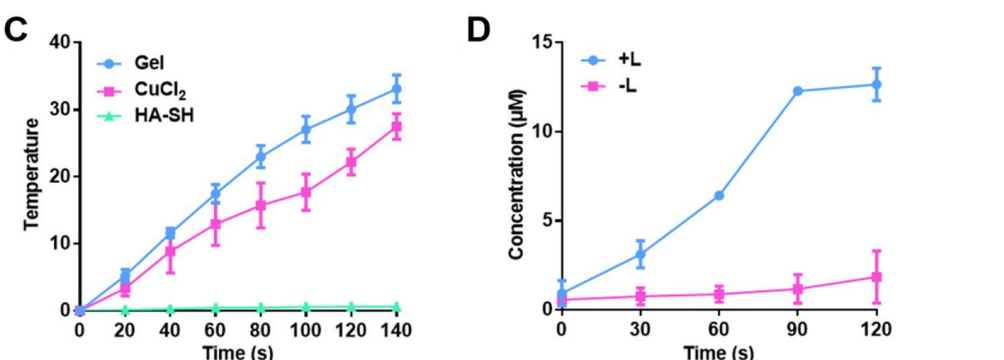
Figure 1. C.Temperature variation curve of hydrogel, CuCl 2 solution and HA-SH solution with NIR laser irradiation. D Release profles of NO from hydrogel with or without NIR laser irradiation
Novel Aspect: The ability of the hydrogel to generate heat upon NIR irradiation provides a strategic advantage over traditional systems, facilitating localized treatment while minimizing systemic side effects.
3. In Vitro Cytotoxicity Assays
Primary Technique: The cytotoxic effects of the hydrogel on 4T1 breast cancer cells were evaluated using MTT assays.
Key Steps:
- Cell Seeding: 4T1 cells were seeded in 96-well plates at a density of 1.0 × 10⁵ cells/well and allowed to adhere overnight.
- Treatment Application: Cells were treated with varying concentrations of the hydrogel and subjected to NIR irradiation for 1 minute. Control groups were maintained without treatment.
Data Collection and Analysis: The viability of the cells was assessed after 24 hours by adding MTT solution, followed by solubilization of the formazan crystals. Absorbance was measured at 492 nm.
Result: The hydrogel treatment resulted in a dose-dependent decrease in cell viability, with the treated group showing approximately 30% viability compared to controls.
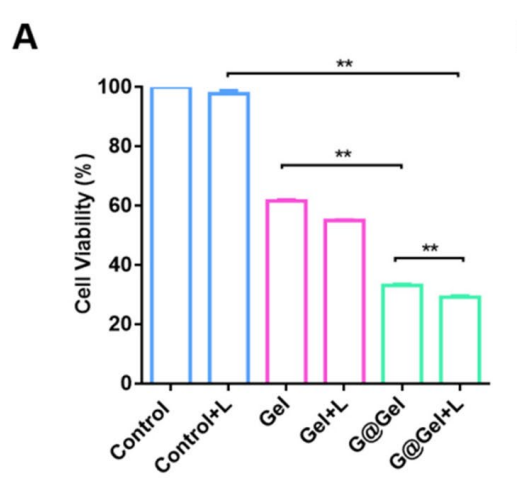
Figure 2. A Cell viability of 4T1 cells with diferent treatments.
Novel Aspect: This study highlights the synergistic effect of combining photothermal therapy with chemodynamic treatment, providing a more effective cancer cell kill rate than conventional single-modal therapies.
4. Assessment of Reactive Oxygen Species (ROS) Generation
Primary Technique: The generation of reactive oxygen species (ROS) was quantified using a DCFH-DA assay.
Key Steps:
- Cell Treatment: 4T1 cells were treated with the hydrogel for 24 hours, followed by NIR irradiation.
- DCFH-DA Staining: The treated cells were incubated with DCFH-DA for 30 minutes to detect intracellular ROS.
Data Collection and Analysis: Fluorescence microscopy was used to visualize and quantify ROS generation, with images captured at specified wavelengths.
Result: Fluorescence intensity indicated a significant increase in ROS levels in the hydrogel-treated cells, confirming the hydrogel’s ability to induce oxidative stress.
Novel Aspect: The use of a hydrogel to simultaneously induce ROS production and deliver therapeutic agents represents an innovative advancement over conventional ROS-generating systems that often require separate delivery mechanisms.
5. In Vivo Efficacy Studies
Primary Technique: The therapeutic efficacy of the hydrogel was evaluated in a 4T1 tumor-bearing mouse model.
Key Steps:
- Tumor Induction: 4T1 cells were subcutaneously injected into the right flank of BALB/c mice. Once tumors reached approximately 200 mm³, treatments commenced.
- Treatment Administration: Mice were divided into groups receiving saline, hydrogel, or hydrogel with NIR laser irradiation. Tumors were monitored for growth over a 21-day period.
Data Collection and Analysis: Tumor volumes were measured using calipers, and statistical analyses were performed to compare growth rates across treatment groups.
Result: The G/aP@Gel group with laser irradiation exhibited a 76.9% inhibition in tumor growth compared to controls, demonstrating the hydrogel’s effectiveness in vivo.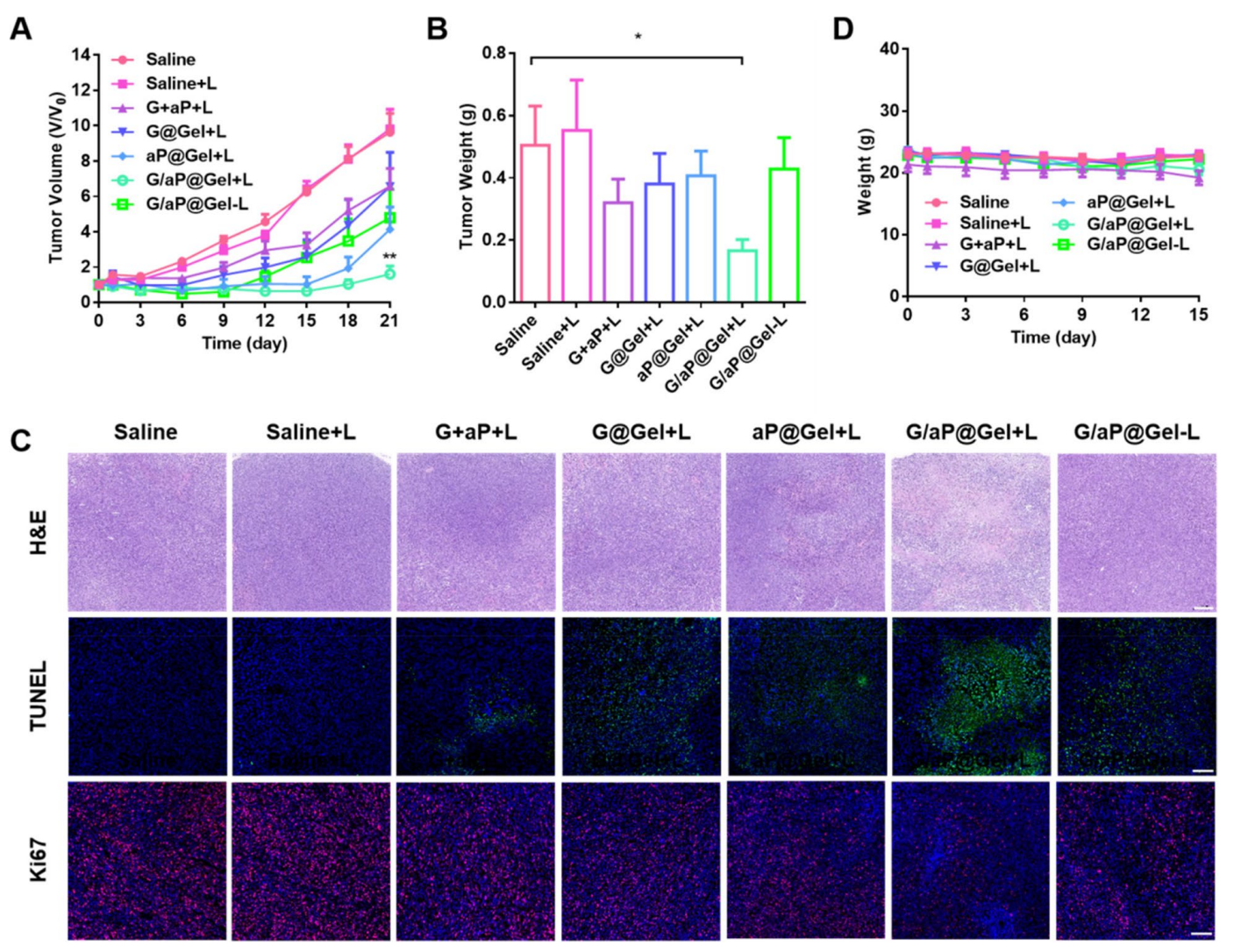
Figure 3.In vivo anti-tumor efects of hydrogel
Novel Aspect: This study showcases the ability of the hydrogel to coordinate multiple therapies (PTT, CDT, and immunotherapy) in a single application, a significant improvement over traditional treatment modalities which typically do not integrate such diverse mechanisms.
Conclusion
The successful development of the copper-induced injectable hydrogel drug delivery system was achieved through the innovative combination of photothermal therapy, chemodynamic therapy, and nitric oxide release, all encapsulated within a biocompatible hydrogel matrix. This multifaceted approach not only enhances localized drug delivery but also actively modulates the tumor microenvironment, addressing the challenges posed by cancer-associated fibroblasts (CAFs) and the immunosuppressive characteristics of tumors.
Key highlights of the study include the demonstration that the hydrogel significantly amplifies immunogenic cell death in tumor cells, leading to increased infiltration of cytotoxic T cells and enhanced anti-tumor immune responses. The hydrogel also effectively depletes CAFs and reduces the differentiation of monocytes into M2-type macrophages, reversing the immunosuppressive tumor microenvironment. Furthermore, the ability of the hydrogel to sustain the release of nitric oxide and immune checkpoint inhibitors like anti-PD-L1 underscores its potential as a robust platform for cancer immunotherapy. This research provides a promising strategy to improve treatment outcomes for patients with cancer, paving the way for future clinical applications.
Reference
Shen, Shuilin, et al. “Copper-induced injectable hydrogel with nitric oxide for enhanced immunotherapy by amplifying immunogenic cell death and regulating cancer-associated fibroblasts.” Biomaterials Research, vol. 27, no. 44, 2023. https://doi.org/10.1186/s40824-023-00389-4.
