Editor: Sarah
In recent years, precision medicine has emerged as a cornerstone of cancer treatment, focusing on tailoring therapies based on individual patient characteristics. A novel study introduces an innovative approach to localized drug delivery that could significantly enhance the precision and effectiveness of cancer treatments. Researchers have developed pH-responsive nanofiber buttresses, a new class of materials designed to deliver drugs directly to tumor sites. These nanofibers, created through electrospinning techniques, exhibit a unique ability to transition from a hydrophobic to a hydrophilic state when exposed to acidic conditions, which mimic the environment found in tumor tissues. This property makes them especially well-suited for targeted drug release at the tumor site, where the extracellular pH typically ranges from 5.5 to 6.5.
Addressing Challenges in Drug Delivery
A longstanding challenge in cancer treatment is the ability to precisely target drug delivery to tumor sites while minimizing systemic side effects. Traditional drug delivery methods often lead to unwanted side effects due to systemic drug distribution, which can also reduce the bioavailability of the drug at the target site. The pH-responsive nanofiber buttresses developed in this study offer a solution to this challenge by ensuring that drugs are released exclusively in areas with acidic pH, such as tumors, thereby reducing the risk of off-target effects and enhancing therapeutic efficacy. This innovation holds potential for advancing cancer treatments, particularly in surgical oncology, where these materials could facilitate more effective drug delivery during tumor resections.
Overview of Existing pH-Responsive Drug Delivery Systems
Research into stimuli-responsive drug delivery systems has made significant progress over the past decade, with pH-sensitive materials gaining particular attention. These materials, which release their drug payloads in response to changes in pH, are particularly useful in the context of tumors, where the extracellular environment is often more acidic than in healthy tissues. Previous studies have explored various pH-responsive systems, including nanoparticles, hydrogels, and nanofibers. However, many of these systems have struggled with issues such as premature drug release or insufficient control over the rate of release, limiting their effectiveness in clinical applications.
Key Findings of the Study
This study introduces a promising solution to these issues by presenting polymeric, pH-responsive nanofiber buttresses. The nanofibers exhibit several key advantages:
- Controlled Drug Release at pH 5.5: These nanofibers demonstrate controlled drug release at a pH of 5.5, typical of tumor environments. At neutral pH (pH 7.4), however, the drug release is negligible, minimizing the risk of off-target drug effects.
- Effective Cytotoxicity of Released Drugs: Drugs such as docetaxel and doxorubicin remain effective against human cancer cell lines even after being released from the nanofibers. This confirms that the nanofiber system retains the full therapeutic potential of the drugs after release.
- Mechanical Properties for Surgical Applications: The nanofibers exhibit sufficient mechanical strength (tensile strength of 6.3 kPa) to withstand the stresses involved in surgical applications. This makes them suitable not only for drug delivery but also for use as structural supports during surgeries, such as tumor resections.
- Sustained Drug Release: The study demonstrated that docetaxel and doxorubicin can be released over extended periods—up to 20 days for docetaxel and 7 days for doxorubicin—under conditions that simulate the tumor microenvironment, ensuring sustained therapeutic effects.
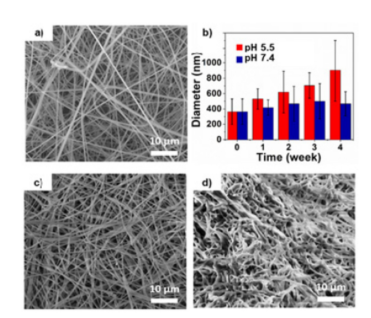
Figure 1: The relationship between the release rate of fluorescein amine and the diameter of nanofibers.
These findings suggest that the pH-responsive nanofiber buttresses have significant potential for use in localized cancer therapy, particularly in surgical settings where continuous and controlled drug release is critical.
Synthesis and Fabrication of pH-Responsive Nanofibers
The nanofiber buttresses were synthesized through electrospinning a polymeric material that contains pH-sensitive side chains. These side chains, consisting of acetal groups, undergo hydrolysis in acidic conditions, which triggers the transition of the fibers from hydrophobic to hydrophilic. The change in the chemical state of the nanofibers leads to fiber swelling and the subsequent release of encapsulated drugs.
In this study, chemotherapeutic agents such as docetaxel and doxorubicin were encapsulated in the nanofibers, and the drug release profiles were evaluated under various pH conditions (pH 5.5, pH 6.5, and pH 7.4). The researchers found that drug release was significantly higher at acidic pH, while the release at neutral pH was minimal, confirming the targeted drug delivery capability of the system.
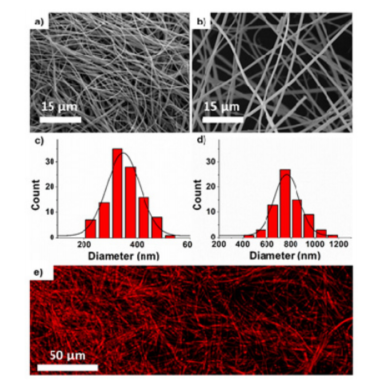
Figure 2: SEM images of nanofibers.
Experimental Validation and Characterization
Several experimental methods were employed to validate the functionality of the pH-responsive nanofibers. These included:
- In Vitro Cytotoxicity Assays: The cytotoxicity of the drug-loaded nanofibers was tested on several human cancer cell lines. The results showed that the drugs retained their cytotoxic activity even after being released from the nanofibers, further validating the system’s potential for targeted cancer therapy.
- Mechanical Testing: The mechanical strength of the nanofibers was assessed through tensile strength testing, which confirmed their suitability for use in surgical applications, where they can function both as drug delivery systems and structural supports during procedures like tumor resections.
- Electron Microscopy and Mass Spectrometry: Techniques such as scanning electron microscopy (SEM) and liquid chromatography-mass spectrometry (LC-MS) were used to thoroughly characterize the nanofibers, ensuring that the drug release mechanisms and the cytotoxicity of the released drugs were accurately measured.
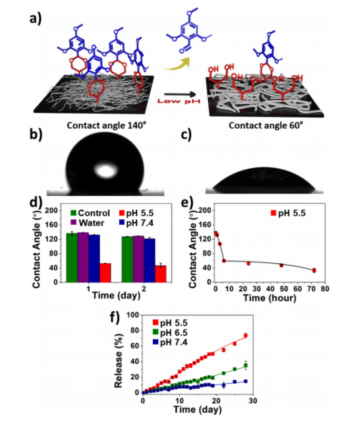
Figure 3: pH response changes and contact angle measurements.
Localized Cancer Therapy and Precision Medicine
The development of pH-responsive nanofiber buttresses represents a significant advancement in the field of localized cancer therapy. By providing both structural support and controlled drug release, these devices offer a promising tool for use in surgical oncology, particularly during tumor resections. They could help ensure that chemotherapy drugs are delivered directly to the tumor site, increasing the efficacy of the treatment while minimizing systemic side effects.
Furthermore, this technology aligns with the principles of precision medicine, where treatments are tailored to the specific characteristics of a patient’s cancer. By ensuring that drugs are delivered exclusively to the tumor, the pH-responsive nanofiber buttresses could enhance the effectiveness of cancer treatments and improve patient outcomes.
Broader Implications for Drug Delivery Systems
The implications of this study extend beyond cancer treatment. The ability to precisely control drug release based on pH conditions has potential applications in a variety of other diseases that involve localized pH changes, such as certain inflammatory conditions. The scalability of the electrospinning process also makes this approach highly promising for clinical use, offering a cost-effective solution for personalized drug delivery.
Future Outlook and Clinical Applications
Future studies will be needed to evaluate the in vivo performance of the pH-responsive nanofiber buttresses, refine their design, and explore their applications in the treatment of other diseases. The continued development of this technology could lead to improved outcomes for cancer patients and those with other conditions where precise drug delivery is essential.
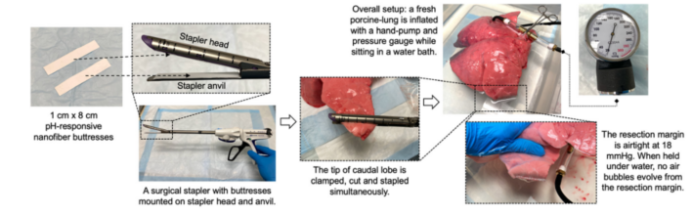
Figure 4: Feasibility testing of nanofibers as surgical reinforcement materials.
Reference
Grinstaff, Mark W., et al. “pH-Responsive Nanofiber Buttresses as Local Drug Delivery Devices.” Biomaterials Science, vol. 11, no. 3, 2023, pp. 813-821. Royal Society of Chemistry, https://doi.org/10.1039/d2bm01199a.
