Editor: Tiffany
A novel lipid nanoparticle system enables adipose stem cells to produce therapeutic proteins sustainably, significantly accelerating wound healing in diabetic mice, offering a promising approach for treating diabetic foot ulcers.
Key Highlights
- Research Question:
Can adipose stem cells (ASCs) be engineered to enhance their tissue repair capability for improved wound healing in diabetic foot ulcers (DFUs) by achieving sustained production of therapeutic proteins? - Research Difficulties:
ASCs exhibit limited protein-producing capacity, and conventional reprogramming methods like electroporation and viral vectors face challenges such as low efficiency, toxicity, or risks of insertional mutagenesis, hindering clinical translation. - Key Findings:
Isomannide-derived lipid nanoparticles (DIMIT LNPs) efficiently deliver self-amplifying RNA (saRNA) and E3 mRNA to ASCs, enabling prolonged expression of hepatocyte growth factor (HGF) and C-X-C motif chemokine ligand 12 (CXCL12), which significantly enhance wound healing in a diabetic mouse model compared to wild-type ASCs. - Innovative Aspects:
The study develops a non-viral LNP platform for ASC engineering, using saRNA and E3 mRNA to overcome immune-mediated translation shutdown, achieving durable protein secretion without compromising ASC phenotypes or requiring viral vectors. - Importance of the Study:
This approach addresses the inefficiencies of current DFU treatments by enabling topical, sustained protein secretion to remodel the wound microenvironment, offering a versatile platform for ASC-based therapies applicable to various diseases.
Diabetic Foot Ulcers and Current Treatment Challenges
Diabetic foot ulcers (DFUs) represent a severe complication of diabetes, significantly reducing patients’ quality of life due to their high prevalence and chronic nature. As a leading cause of non-traumatic lower limb amputations, DFUs affect millions globally, with impaired wound healing being the primary trigger. The pathological process in DFUs involves a failure to progress through normal healing stages, resulting in a chronic, highly inflammatory state that disrupts coordinated local immune crosstalk. This impaired healing is exacerbated by the diabetic condition, which compromises tissue repair mechanisms.
Current therapeutic options for DFUs are limited. Regranex, a recombinant platelet-derived growth factor beta (PDGF-BB) topical gel, is the only U.S. Food and Drug Administration (FDA)-approved standard treatment for DFUs to date. However, its efficacy is restricted, benefiting only approximately 50% of early-stage DFU patients and showing limited effectiveness in pressure or venous stasis ulcers. Other strategies, such as topical dressings and administration of therapeutic proteins, have been explored, but they often fail to adequately remodel the local microenvironment of diabetic wounds, necessitating innovative approaches to enhance therapeutic outcomes.
Objectives to Enhance ASCs for Diabetic Wound Repair
The research addressed critical questions: How can the tissue repair capability of adipose stem cells (ASCs) be enhanced to improve wound healing in DFUs? What are the difficulties in achieving sustained protein production in ASCs for therapeutic purposes? ASCs are promising due to their self-renewal, low immunogenicity, and immunomodulating properties, but their limited protein-producing capacity hinders optimal tissue regeneration. Conventional reprogramming methods, such as electroporation and viral vectors, face challenges like low efficiency, toxicity, or risks of insertional mutagenesis, limiting clinical translation.
The research aimed to develop a lipid nanoparticle (LNP) system for delivering self-amplifying RNA (saRNA) and E3 mRNA to ASCs, enabling sustained production of therapeutic proteins like hepatocyte growth factor (HGF) and C-X-C motif chemokine ligand 12 (CXCL12) to accelerate wound healing. The main objectives were: (a) Synthesize and optimize sugar alcohol-derived LNPs for efficient RNA delivery to ASCs; (b) Engineer ASCs with saRNA and E3 mRNA to achieve sustained protein production; © Evaluate the therapeutic efficacy of engineered ASCs in a diabetic mouse wound model.
The study was conducted by a team led by Yizhou Dong from The Ohio State University and Icahn School of Medicine at Mount Sinai, with key contributions from Yonger Xue, Yuebao Zhang, and Yichen Zhong. The research was published in Nature Communications in 2024.
Experimental Approaches and Key Outcomes
Experimental Procedures
- Synthesis of sugar alcohol-derived ionizable lipids, including isomannide-based (DIM), isosorbide-based (DIS), and L-sorbitol-based (LIS) lipids.
- Formulation of LNPs with these lipids, helper lipids (DOPE, cholesterol, DMG-PEG2k), and RNA cargos.
- Characterization of LNPs for hydrodynamic diameter, zeta potential, and RNA encapsulation efficiency.
- In vitro delivery of RNA to ASCs, assessing protein expression via luminescence and fluorescence assays.
- Engineering ASCs with therapeutic RNA, such as HGF saRNA and E3 mRNA, to produce therapeutic proteins.
- In vivo studies: embedding engineered ASCs in HyStem-HP hydrogels and applying them to diabetic wounds in db/db mice, followed by monitoring wound closure over time.
Key Experiments
a. Optimization of LNP formulations for RNA delivery to ASCs
- Procedure: Various sugar alcohol-derived LNPs were tested for their ability to deliver firefly luciferase (FLuc) mRNA to primary murine ASCs, with delivery efficiency measured by luminescence intensity. An L16 (4^4) orthogonal table guided formulation optimization, adjusting molar ratios of lipids and mass ratios of lipid to mRNA.
- Result: Isomannide-based DIMIT LNPs exhibited the highest delivery efficiency, over 70-fold more effective than Lipofectamine 3000 and electroporation, and 140-fold greater than FDA-approved ALC-0315 and MC3 LNPs. DIMIT LNPs achieved over 90% GFP-positive ASCs when delivering GFP mRNA.
- Finding: DIMIT LNPs are highly effective for RNA delivery to ASCs, surpassing conventional methods and FDA-approved LNP formulations, making them ideal for ASC engineering.
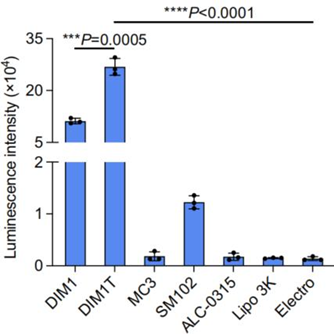
Figure 1. Luminescence intensity of DIM1T LNPs and other control groups.
b. Investigation of saRNA expression dynamics and the role of E3 mRNA
- Procedure: ASCs were treated with DIMIT LNPs encapsulating FLuc saRNA alone or combined with E3 mRNA (termed SEC). Protein expression was monitored over 9 days, and phosphorylation levels of protein kinase R (PKR) and eukaryotic initiation factor 2 alpha (eIF2α) were assessed to investigate immune responses.
- Result: FLuc saRNA alone showed a sharp decline in expression after 48 hours, attributed to dsRNA-triggered PKR and eIF2α phosphorylation, which terminates translation. Co-delivery of E3 mRNA with saRNA sustained FLuc expression for over 9 days, with significantly reduced phosphorylation levels.
- Finding: E3 mRNA mitigates the immune response triggered by saRNA, preventing translation shutdown and enabling sustained protein expression in ASCs, critical for therapeutic applications.
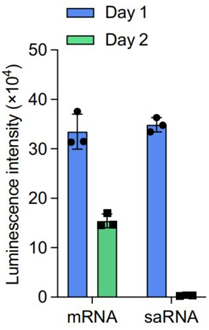
Figure 2. Time-course luminescence of FLuc saRNA versus FLuc SEC (saRNA + E3 mRNA) in ASCs.
c. In vivo wound healing assay in diabetic mice
- Procedure: ASCs were engineered with HGF saRNA and E3 mRNA using DIMIT LNPs (termed DS-ASCs), embedded in HyStem-HP hydrogels, and applied to full-thickness wounds in db/db mice. Wound closure was monitored every 3 days until full re-epithelialization, with wound size normalized against initial areas.
- Result: DS-ASCs significantly accelerated wound healing compared to wild-type ASCs, HGF mRNA-treated ASCs, or vehicle controls. By day 18, the average wound size in the HGF DS-ASC group was 0.00 ± 0.00 mm², with the lowest area under the curve (AUC) among all groups.
- Finding: Engineered ASCs with sustained HGF production markedly enhance wound healing in a diabetic mouse model, demonstrating the therapeutic potential of the LNP-RNA platform.
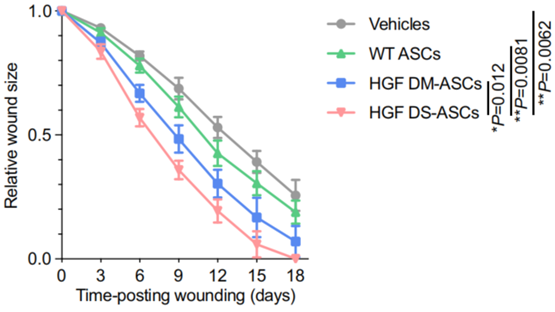
Figure 3. Relative wound size over time in db/db mice treated with HGF DS-ASCs.
Impact and Future Directions
The study developed DIMIT LNPs as an effective platform for delivering saRNA and E3 mRNA to ASCs, achieving sustained production of therapeutic proteins like HGF and CXCL12 for over 9 days. In a diabetic mouse model, DS-ASCs embedded in hydrogels significantly accelerated wound closure compared to wild-type ASCs or vehicle controls, with complete healing observed by day 18. The innovation lies in overcoming the limitations of transient protein expression in ASCs by using a non-viral LNP system that avoids the risks of viral vectors while ensuring high delivery efficiency and biocompatibility. This approach addresses the inefficiencies of current DFU treatments, such as Regranex, by enabling topical, sustained protein secretion to remodel the wound microenvironment. The significance of this research extends beyond DFUs, offering a versatile platform for engineering ASCs to treat various diseases, potentially transforming regenerative medicine and cell-based therapies.
Reference:
Xue, Yonger, et al. “LNP-RNA-engineered adipose stem cells for accelerated diabetic wound healing.” Nature Communications 15.1 (2024): 739.
