Editor: Nina
Researchers develop nanoengineered injectable hydrogels incorporating layered double hydroxides and alginate for sustained release of recombinant human erythropoietin to enhance treatment outcomes in patients with chronic kidney disease-related anemia.
Key Preview
Research Question
The study addresses the challenge of frequent administration of recombinant human erythropoietin (rhEPO) in patients with Chronic Kidney Disease (CKD), which is characterized by a deficiency of the glycoprotein. The current treatment requires multiple injections due to the short half-life of rhEPO, leading to potential side effects.
Research Design and Strategy
The researchers designed nanoengineered injectable hydrogels that incorporate layered double hydroxides (LDHs) into alginate gels to control the release of rhEPO. This approach aims to enhance therapeutic efficacy while reducing the frequency of administration.
Method
The study utilized a combination of in vitro and in vivo experiments, including gelation time assessment, mechanical testing, EPO release kinetics, and biocompatibility assays, to evaluate the performance of the injectable hydrogels.
Key Results
The newly developed injectable hydrogels demonstrated a significant reduction in initial burst release, with only 24% of rhEPO released over 108 hours compared to 86% from conventional alginate gels. In vivo studies confirmed the gels’ stability without adverse effects.
Significance of the Research
This research presents a significant advancement in drug delivery systems for protein therapeutics, particularly for CKD patients, offering a minimally invasive option that could improve patient compliance and therapeutic outcomes.
Introduction
Chronic Kidney Disease (CKD) is a progressive condition characterized by a gradual decline in kidney function, which can ultimately lead to kidney failure. One of the significant complications associated with CKD is anemia, primarily due to insufficient production of erythropoietin (EPO), a glycoprotein hormone that stimulates red blood cell production in the bone marrow. As kidney function deteriorates, EPO levels drop, resulting in decreased erythropoiesis and subsequently leading to anemia, fatigue, and reduced quality of life for affected individuals. The management of anemia in CKD patients has become a crucial aspect of therapy to improve patient outcomes and overall health.
The current standard treatment for anemia in CKD involves the administration of recombinant human erythropoietin (rhEPO), which is typically delivered via intravenous or subcutaneous injections. However, this traditional approach presents several challenges. The pharmacokinetics of rhEPO are characterized by a short half-life, necessitating frequent injections—often 1 to 3 times per week—to maintain effective therapeutic levels. This frequent administration not only poses a burden on patients but can also lead to fluctuating EPO levels in the bloodstream, which may result in adverse effects such as hypertension, thromboembolic events, and increased mortality risks. Consequently, the need for a more efficient and patient-friendly drug delivery system is evident.
To address these challenges, researchers are exploring innovative drug delivery strategies that aim to enhance the efficacy and safety of protein therapeutics like rhEPO. One promising approach is the development of nanoengineered injectable hydrogels, which can encapsulate drugs and provide controlled release over extended periods. By incorporating materials such as layered double hydroxides (LDHs) into alginate-based hydrogels, the release profile of rhEPO can be modulated, reducing the frequency of injections while minimizing side effects. This novel strategy presents a significant advancement in drug delivery systems, offering the potential for improved patient compliance and therapeutic outcomes in the management of CKD-related anemia.
Research Team and Aim
The research team comprises a diverse group of experts in the fields of biomaterials and nanotechnology. The study was led by Dr. V. H. Giang Phan, alongside prominent researchers such as Hai-Sang Duong, Quynh-Giao Thi Le, and others from various institutions, including Ton Duc Thang University and New York University. The research was conducted between 2022 and 2023 and culminated in the publication of the paper titled “Nanoengineered injectable hydrogels derived from layered double hydroxides and alginate for sustained release of protein therapeutics in tissue engineering applications,” which appeared in the Journal of Nanobiotechnology.
The primary aim of the research, as articulated by Dr. V. H. Giang Phan, was to “develop and characterize injectable hydrogels that enhance the controlled release of rhEPO, ultimately improving treatment protocols for patients with Chronic Kidney Disease (CKD).” This objective reflects the team’s commitment to addressing the challenges associated with traditional drug delivery systems and improving therapeutic outcomes for CKD patients.
Experimental Process
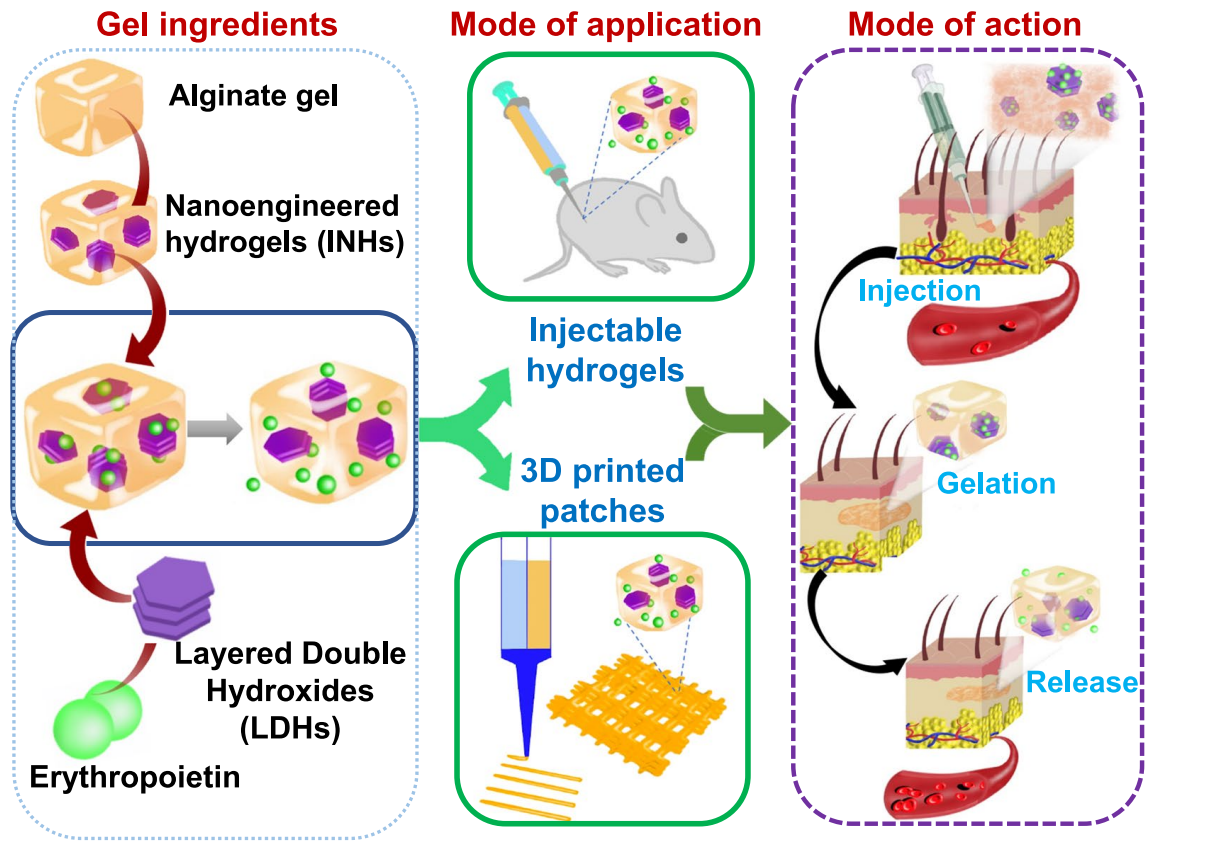
Scheme 1. Working principle of INHs. Preparation of the EPOloaded INHs from alginate gel and LDHs and their utilization in injectable hydrogel and 3D printing applications. Efect of sustained release on tissue regeneration after in situ implantation of INHs at the subcutaneous tissue of mice.
Primary Technique
The primary technique employed in this study was the development of nanoengineered injectable hydrogels (INHs) utilizing layered double hydroxides (LDHs) integrated into sodium alginate (SA). This approach aimed to create a controlled release system for recombinant human erythropoietin (rhEPO) which addresses the limitations of conventional drug delivery methods.
Key Steps
- Synthesis of LDHs:
- Materials: Magnesium chloride hexahydrate (MgCl2.6H2O) and aluminum chloride hexahydrate (AlCl3.6H2O) were used as precursors.
- Procedure: The two salts (6.82 g of MgCl2.6H2O and 4.04 g of AlCl3.6H2O) were dissolved in 100 mL of deionized water. The pH of the solution was adjusted to 9.0 using 1 M NaOH, followed by magnetic stirring at 80 °C for 48 hours.
- Outcome: The resulting LDH nanoparticles were collected through centrifugation, washed, and dried at 60 °C to yield a white powder.
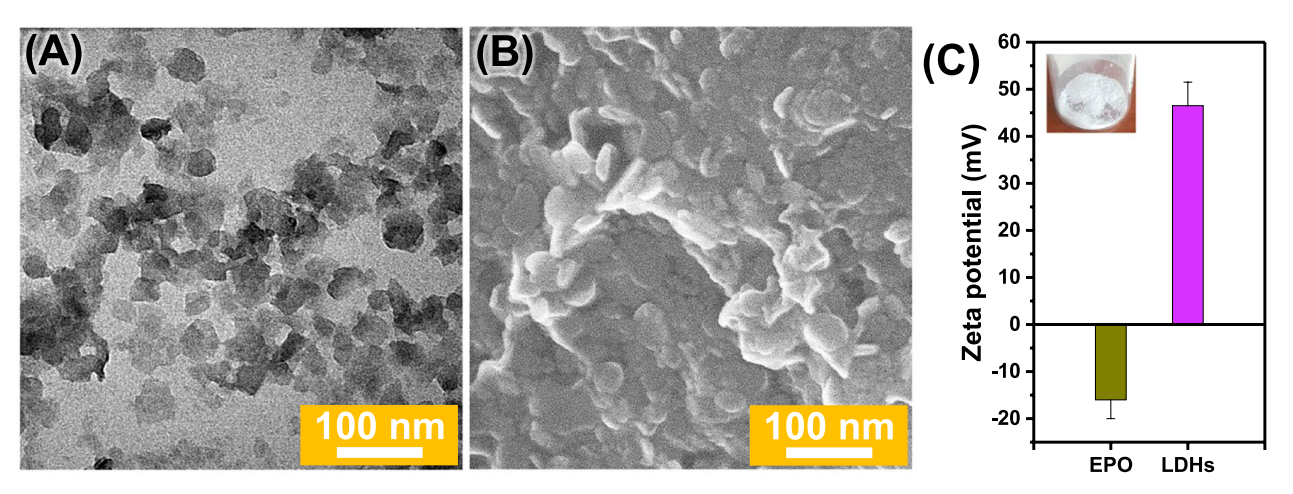
Figure 1. A TEM image of LDHs. B SEM image of LDHs. C Zeta potential of EPO and LDHs dispersed in deionized water. Inset photograph is for the dried LDHs.
- Gelation Time Assessment:
- Materials: A 4% (w/v) SA solution was prepared, and various concentrations of calcium ions (Ca2+) ranging from 0.005 to 0.03 mol/L were used.
- Procedure: The optimal gelation time was determined by mixing SA with different Ca2+ concentrations and measuring the time taken for gel formation using a stopwatch.
- Outcome: The ideal concentration of 0.02 mol/L Ca2+ was identified, facilitating the subsequent formation of INHs.
- Preparation of Injectable Hydrogels:
- Materials: The previously synthesized LDHs and SA were used to develop the hydrogels.
- Procedure: Combinations of SA (4% w/v) and LDHs (0.125, 0.25, and 0.5 mg/mL) were mixed, then crosslinked with the optimal Ca2+ concentration (0.02 mol/L) to form the INHs.
- Outcome: Formulated hydrogels were characterized in terms of their injectability and mechanical properties.
- Mechanical Testing:
- Procedure: Compressive strength tests were conducted using a Universal Testing Machine at a crosshead speed of 10 mm/min to assess the hydrogels’ mechanical properties.
- Outcome: The compressive strength of INHs was significantly higher (1.75 MPa for INHs-0.25) compared to Alg-Gel (0.056 MPa), showcasing improved mechanical integrity.
- In Vitro Release Studies:
- Materials: EPO was encapsulated in both Alg-Gel and the INHs for comparison.
- Procedure: Hydrogels were placed in PBS at 37 °C, and samples were taken at various time points (2, 4, 6, 12, 18, 24, 36, 48, 60, 72, 84, and 108 hours) to measure EPO release using the Bradford protein assay.
- Outcome: The INHs demonstrated a controlled release profile, with only 30% of EPO released over 132 hours compared to 100% from conventional Alg-Gel.
- Biocompatibility Tests:
- Procedure: MTT assays were conducted using HaCaT keratinocytes and BLO-11 mouse muscle fibroblast cells to assess cell viability on the hydrogels after 48 hours of incubation.
- Outcome: Cell viability remained above 80%, confirming the biocompatibility of the hydrogels.
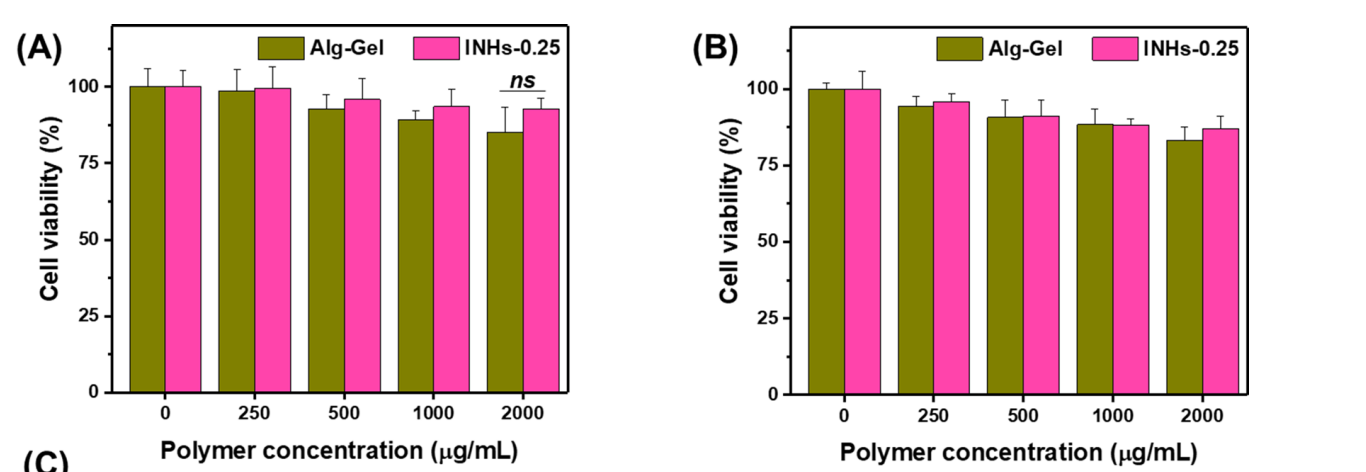
Figure 2. In vitro biocompatibility test. A and B Biocompatibility of HaCaT keratinocytes and BLO11 mouse muscle fbroblast cells incubated with hydrogel formulation for 24 h.
- In Vivo Studies:
- Procedure: Albino mice were used to evaluate the injectability and stability of the hydrogels. The hydrogels were implanted subcutaneously, and after 30 minutes, the mice were sacrificed to assess gel formation.
- Outcome: INHs formed stable gels in vivo without adverse effects, confirming their potential for clinical application.
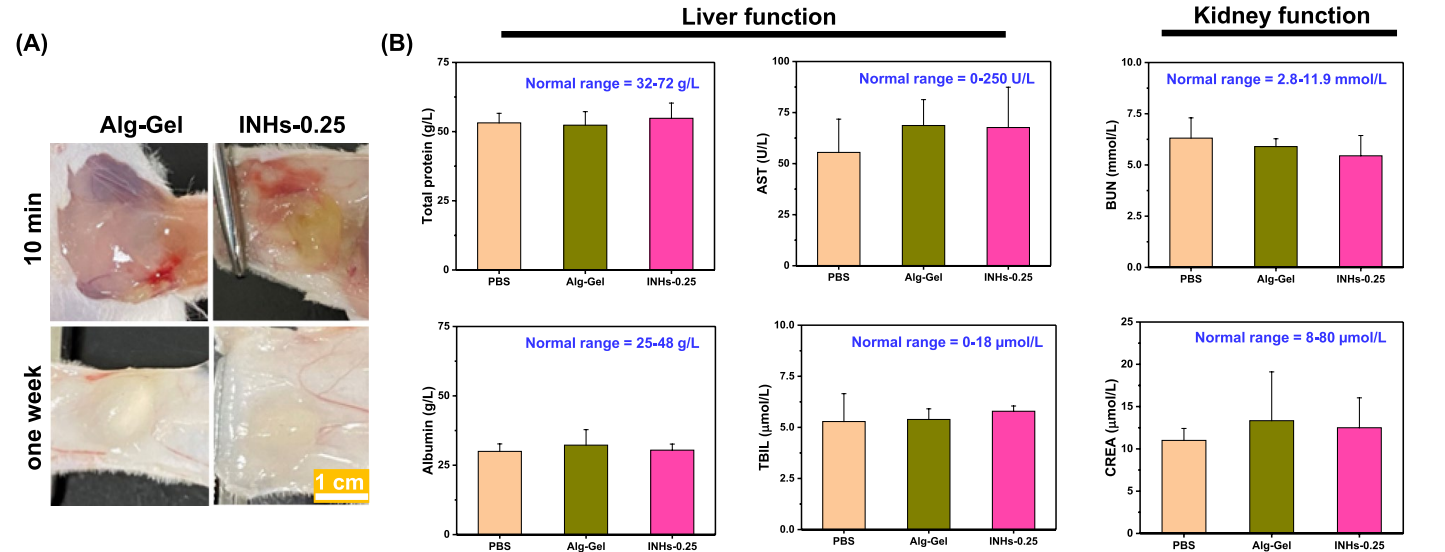
Figure 3. In vivo safety of INHs. A In vivo biodegradation of INHs and AlgGel. To examine biodegradation, hydrogels (100 µL, 4 wt.%) were injected into the back of mice and recovered after one week. B Blood biochemistry analysis of mice implanted with hydrogels one week after injection. AST: aspartate transaminase; TBIL Total bilirubin, BUN Blood urea nitrogen; and CREA creatinine.
Data Collection and Analysis
Data were collected through quantitative assays such as MTT for cell viability, Bradford for protein quantification, and mechanical testing for compressive strength. Statistical analyses were performed using one-way ANOVA with Tukey’s post hoc test to evaluate the significance of the results (p < 0.05) using OriginPro 9.0 software.
Results
The key results indicated that INHs exhibited:
- A significant reduction in initial burst release of EPO (only 30% released over 132 hours).
- Enhanced mechanical properties (compressive strength of 1.75 MPa).
- High biocompatibility with cell viability over 80%.
- Successful gel formation in vivo without adverse effects.
Novel Aspects
The study introduced the innovative use of LDHs in alginate-based hydrogels, which is a departure from traditional drug delivery systems. The incorporation of LDHs not only improved the mechanical strength but also allowed for controlled and sustained release of protein therapeutics like rhEPO, thereby potentially reducing the frequency of injections and enhancing patient compliance. This advancement underscores the versatility and efficacy of the newly developed INHs, making them a promising candidate for clinical applications in tissue engineering and regenerative medicine.
Conclusion
The successful development of the nanoengineered injectable hydrogels (INHs) presented in this study is achieved through a combination of innovative material design and strategic incorporation of layered double hydroxides (LDHs) into alginate-based hydrogels. This approach not only enhances the mechanical properties of the hydrogels but also allows for a controlled and sustained release of recombinant human erythropoietin (rhEPO), significantly addressing the shortcomings of traditional drug delivery systems.
The highlights of the study include the significant reduction in the initial burst release of EPO, with only 30% released over 132 hours compared to 100% from conventional alginate gels. Additionally, the INHs demonstrated enhanced mechanical strength (compressive strength of 1.75 MPa), high biocompatibility with cell viability above 80%, and the ability to form stable gels in vivo without adverse effects. These findings indicate that the proposed INHs represent a promising advancement in drug delivery systems for CKD management, offering a minimally invasive option that could improve patient compliance and therapeutic outcomes. Ultimately, this research lays the groundwork for future applications in tissue engineering and regenerative medicine, showcasing the potential of this innovative delivery platform for various protein therapeutics.
Reference
Phan, V. H. Giang, et al. “Nanoengineered Injectable Hydrogels Derived from Layered Double Hydroxides and Alginate for Sustained Release of Protein Therapeutics in Tissue Engineering Applications.” Journal of Nanobiotechnology, vol. 21, no. 405, 2023, doi:10.1186/s12951-023-02160-
