Editor: Tiffany
A new study introduces a nanotechnology-based formulation that combines green-synthesized iron oxide nanoparticles with oleanolic acid to enhance targeted drug delivery for lung cancer, showing significant cytotoxic effects on cancer cells while raising important considerations for biosafety.
Key Highlights
- Research Question:
Can solid lipid nanoparticles loaded with oleanolic acid enhance the cytotoxic effects against human lung carcinoma cells while maintaining an acceptable biosafety profile? - Research Difficulties:
The study faced challenges in optimizing doses of the nanoparticles to ensure efficacy against cancer cells without adversely affecting normal bronchial tissues. - Key Findings:
SLN samples significantly reduced the viability of A549 lung cancer cells to under 30% and adversely affected bronchial microtissues, indicating a need to adjust dosages for improved safety. - Innovative Aspects:
The research utilized green iron oxide nanoparticles synthesized from plant extracts, showcasing an eco-friendly approach to drug delivery systems. - Importance of the Study:
The findings contribute to the growing field of nanotechnology in medicine, highlighting the potential for improved lung cancer treatments through targeted drug delivery mechanisms.
Challenges in Lung Cancer Treatment and the Need for Innovative Solutions
Lung cancer remains a formidable global health challenge, recognized as the second most common type of cancer worldwide while holding the unenviable position of the leading cause of cancer-related mortality. Within this broad category, non-small cell lung cancer (NSCLC) emerges as a predominant subtype, accounting for a significant proportion of cases and posing substantial therapeutic difficulties. As reported in a 2024 study published in Medicina, “Lung cancer represents the second most common type of cancer worldwide, but it is the first type in the leading causes of cancer-related deaths”. This statistic underscores the urgent need for effective interventions.
The symptomatic presentation of lung cancer, though not explicitly detailed in the referenced paper, typically includes persistent cough, shortness of breath, chest pain, hoarseness, unexplained weight loss, and hemoptysis (coughing up blood). These symptoms often manifest in advanced stages, complicating timely diagnosis and treatment. The paper highlights the array of conventional therapeutic strategies available, which include surgery, chemotherapy, and radiotherapy. For patients with advanced metastatic disease, options expand to encompass targeted therapy and immunotherapy. Despite advancements in these modalities, significant limitations persist, as noted: “Although new advances in the treatment of lung cancer have been developed, new treatments for patients suffering from end-stage lung cancer and beyond are still needed.” Key challenges include suboptimal drug delivery to tumor sites, systemic side effects due to non-specific targeting, and reduced efficacy in late-stage disease, all of which drive the pursuit of innovative solutions.
Developing a Novel Nano-Enabled Formulation for Targeted Lung Cancer Therapy
Addressing these therapeutic shortcomings, a research team spearheaded by C.P.-B. and E.-A.M. from the “Victor Babes” University of Medicine and Pharmacy in Timisoara, Romania, set out to pioneer a novel approach. Their study, detailed in a 2024 Medicina publication, aimed to enhance lung cancer treatment through the development of a nano-enabled pharmaceutical formulation. Specifically, the researchers sought to engineer solid lipid nanoparticles (SLNs) incorporating oleanolic acid (OA) and green-synthesized iron oxide nanoparticles (IONPs) to achieve targeted drug delivery. The paper articulates this objective: Therefore, the development of enabled pharmaceutical nanoparticles has the potential to be used for targeted drug release, leading to an increased effect of chemotherapy in lung cancer”.
The motivation stemmed from the recognized deficiencies in current treatments, such as poor drug localization and adverse effects on healthy tissues. By leveraging nanotechnology, the team aimed to improve the precision and efficacy of drug administration while mitigating systemic toxicity. The use of green-synthesized IONPs, derived from plant extracts of Camellia sinensis (green tea) and Ocimum basilicum (basil), further aligned the research with sustainable methodologies, enhancing the potential biocompatibility of the nanoparticles.
Synthesis, Characterization, and Biological Evaluation of SLNs with Green IONPs and OA
Experimental Procedures
The study involved the following key experimental steps:
- Preparation of green iron oxide nanoparticles (IONPs) using alcoholic extracts from Camellia sinensis (green tea) and Ocimum basilicum (basil) at 25°C and 80°C.
- Fabrication of solid lipid nanoparticles (SLNs) by loading the green IONPs with oleanolic acid (OA) via the emulsification-diffusion method.
- Characterization of the SLNs using Fourier Transform Infrared (FTIR) spectroscopy, Transmission Electron Microscopy (TEM), and Dynamic Light Scattering (DLS) to assess composition, size, and stability.
- Quantification of entrapment efficiency (EE%) and drug loading capacity (DLC%) of OA in the SLNs using High-Performance Liquid Chromatography (HPLC).
- In vitro biosafety evaluation on 3D bronchial microtissues (EpiAirway™ model) using the MTT assay.
- Cytotoxicity assessment on A549 human lung carcinoma cells using the Alamar blue assay.
Key Experiments
1. Synthesis of Solid Lipid Nanoparticles (SLNs)
- Procedure: SLNs were synthesized using the emulsification-diffusion method. An organic phase was prepared by dissolving phosphatidylcholine in ethanol and mixing it with another organic phase containing green IONPs, glyceryl monostearate, and OA in acetone. This was added to an aqueous phase containing Span 80. The mixture was evaporated to remove organic solvents and sonicated to form SLNs. Four formulations were produced: SLN_CS 25@OA, SLN_CS 80@OA, SLN_OB 25@OA, and SLN_OB 80@OA, based on the plant extract and synthesis temperature of the IONPs.
- Result: Four SLN variants were successfully obtained: SLN_CS 25@OA, SLN_CS 80@OA, SLN_OB 25@OA, and SLN_OB 80@OA, each loaded with OA and green IONPs.
- Finding: The emulsification-diffusion method effectively produced OA-loaded SLNs with green IONPs, establishing a basis for subsequent characterization and biological evaluation.
2. Characterization of SLNs Using TEM and DLS
- Procedure: TEM was used to examine the size and morphology of the SLNs, while DLS measured their hydrodynamic diameter (Hd), polydispersity index (PDI), and zeta potential (ζ-potential).
- Result: TEM showed nearly spherical SLNs with sizes ranging from 7.26 nm to 15.84 nm. DLS revealed hydrodynamic diameters of 66.5 nm to 78.2 nm, PDI values from 0.189 to 0.366, and zeta potentials from -20.7 mV to -38.5 mV.
- Finding: The SLNs displayed small, uniform sizes and high colloidal stability (indicated by negative zeta potentials), making them suitable for drug delivery applications.
3. Determination of Entrapment Efficiency (EE%) and Drug Loading Capacity (DLC%)
- Procedure: Free OA in the SLN formulations was quantified via HPLC. EE% and DLC% were calculated using the following equations:
- EE% = [(OA_total – OA_free) / OA_total] × 100%
- DLC% = [OA_entrapped / m_total lipids] × 100%
- Result: EE% ranged from 80.45% to 93.62%, with the highest values for SLN_OB 25@OA (90.43%) and SLN_OB 80@OA (93.62%). DLC% ranged from 1.226% to 1.427%, also higher for Ocimum basilicum-based SLNs.
- Finding: The SLNs, especially those with Ocimum basilicum-derived IONPs, exhibited high OA entrapment and loading efficiency, suggesting effective drug incorporation.
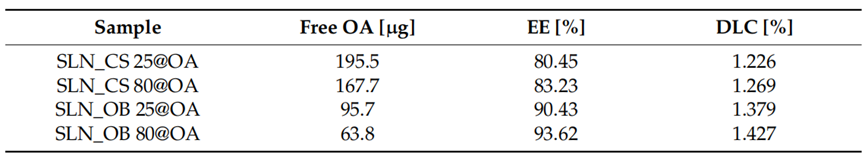
Table 1. EE% and DLC% of OA in the SLNs based on OA-loaded green IONPs.
4. Biosafety Evaluation on EpiAirway™ Microtissues
- Procedure: 3D bronchial microtissues (EpiAirway™ model) were exposed to SLNs at 500 μg/mL (green IONPs concentration) for 24 hours. Tissue viability was assessed using the MTT assay.
- Result: Viability was significantly reduced, with SLN_OB 25@OA and SLN_OB 80@OA showing the lowest rates at 25.54% and 13.93%, respectively, compared to 50.30% for SLN_CS 25@OA and 33.35% for SLN_CS 80@OA.
- Finding: The SLNs demonstrated considerable toxicity to healthy bronchial tissue, indicating potential safety issues that require further investigation.
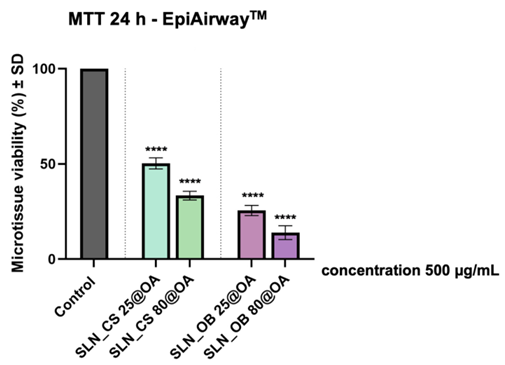
Figure 1. Viability of EpiAirway™ microtissues after 24-hour exposure to SLN formulations at 500 μg/mL green IONPs (MTT assay).
5. Cytotoxicity Assessment on A549 Human Lung Carcinoma Cells
- Procedure: A549 cells were treated with SLNs at concentrations of 500 μg/mL green IONPs and 25 μg/mL OA for 24 hours. Cell viability was measured using the Alamar blue assay.
- Result: SLN_OB 25@OA and SLN_OB 80@OA reduced viability to 28.38% and 18.24%, respectively, while SLN_CS 25@OA and SLN_CS 80@OA reduced it to approximately 50%.
- Finding: The SLNs, particularly those with Ocimum basilicum extracts, showed potent cytotoxicity against A549 lung cancer cells, suggesting their potential as anticancer agents.
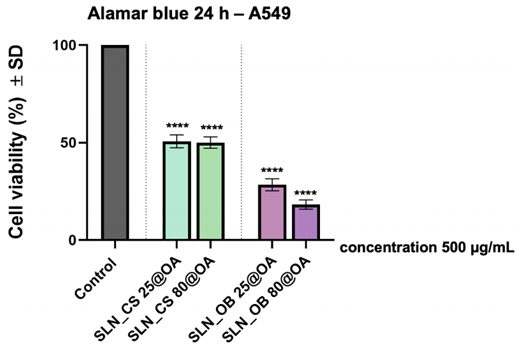
Figure 2. Viability of A549 cells after 24-hour treatment with SLN formulations at 500 μg/mL green IONPs and 25 μg/mL OA (Alamar blue assay).
Implications for Lung Cancer Therapy and Future Research Directions
This study marks a significant advancement in lung cancer therapeutics through the innovative application of nanotechnology. The research team developed SLNs that integrate the antitumor agent oleanolic acid with green-synthesized IONPs, achieving notable cytotoxicity against A549 lung cancer cells. The Ocimum basilicum-derived SLNs, in particular, reduced cancer cell viability to as low as 18.24%, underscoring their therapeutic potential. The use of plant-based synthesis methods not only enhances environmental sustainability but also leverages the bioactive properties of the extracts, potentially improving the nanoparticles’ biocompatibility.
However, the study also revealed challenges, notably the significant reduction in viability of 3D bronchial microtissues, which raises biosafety concerns at the tested concentrations. As the paper concludes, “The findings reported could be important contributions to the development of a new and innovative lung cancer therapeutic approach.” While the SLNs offer a promising avenue for enhancing drug delivery and treatment efficacy, further optimization is essential to balance therapeutic benefits against potential toxicity to healthy tissues. This research lays a robust foundation for future investigations aimed at refining this nanotechnology-based strategy, with the ultimate goal of improving outcomes for patients with NSCLC and beyond.
Reference:
Prodan-Bărbulescu, Cătălin, et al. “A Preliminary Report Regarding the Morphological Changes of Nano-Enabled Pharmaceutical Formulation on Human Lung Carcinoma Monolayer and 3D Bronchial Microtissue.” Medicina 60.2 (2024): 208.
