Editor: Nina
Scientists develop a hyaluronic acid hydrogel containing resveratrol-loaded chitosan nanoparticles to enhance therapeutic efficacy and reduce inflammation in the treatment of atopic dermatitis.
Key Preview
- Research Question: Can a hyaluronic acid hydrogel containing resveratrol-loaded chitosan nanoparticles improve the treatment of atopic dermatitis (AD)?
- Research Design and Strategy: The study focuses on developing a drug delivery system using nanoparticles to enhance the bioavailability of resveratrol, a natural anti-inflammatory compound.
- Method: The researchers synthesized chitosan nanoparticles loaded with resveratrol, incorporated them into hyaluronic acid hydrogels, and evaluated their physicochemical properties and therapeutic effects in vitro.
- Key Results: The resveratrol-loaded nanoparticles demonstrated a high encapsulation efficiency and significantly reduced oxidative stress and inflammatory cytokine secretion in human keratinocytes.
- Significance of the Research: This novel approach presents a promising, biocompatible method for treating AD, potentially improving patients’ quality of life through effective topical delivery of therapeutic agents.
Introduction
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disorder characterized by symptoms such as intense itching, erythema, and the formation of lesions. This multifaceted condition often begins in childhood and can persist into adulthood, significantly impacting patients’ quality of life. The underlying pathophysiology of AD involves a complex interplay of genetic, immunological, and environmental factors, leading to a compromised skin barrier and heightened susceptibility to infections and irritants. As a result, managing AD presents a substantial challenge for both patients and healthcare providers.
Traditionally, treatment strategies for AD have centered around the use of topical corticosteroids and other anti-inflammatory agents. These commonly prescribed medications aim to alleviate symptoms by reducing inflammation and controlling itching. However, prolonged use of steroidal treatments raises concerns about potential side effects, including skin thinning, telangiectasia, and systemic absorption that may lead to other health complications. Furthermore, the efficacy of conventional therapies can be limited by the poor bioavailability of active compounds and the inability to achieve targeted delivery to affected skin areas.
The challenges associated with current treatment approaches result in a cycle of inadequate symptom management and increased reliance on medications, which may lead to adverse effects and diminished patient adherence to prescribed treatment regimens. Consequently, there is a pressing need for innovative drug delivery systems that can enhance the therapeutic efficacy of natural anti-inflammatory agents while minimizing side effects.
In response to these challenges, the present study explores an innovative drug delivery strategy utilizing hyaluronic acid (HA) hydrogels embedded with resveratrol-loaded chitosan nanoparticles. This novel approach aims to improve the bioavailability and sustained release of resveratrol, a natural compound known for its potent anti-inflammatory and antioxidant properties. By integrating resveratrol into a biocompatible hydrogel matrix, this strategy seeks to provide a more effective and targeted therapeutic option for patients suffering from atopic dermatitis, ultimately enhancing their quality of life and reducing the burden of conventional treatments.
Research Team and Aim
The research team for this study comprised a collaborative group of experts in the fields of biomaterials, pharmacology, and dermatology. The lead researcher, Raffaele Conte, spearheaded this investigation alongside Ilenia De Luca, Anna Valentino, Pierfrancesco Cerruti, Parisa Pedram, Gustavo Cabrera-Barjas, Arash Moeini, and Anna Calarco. This research was conducted in 2023 at various esteemed institutions, including the Research Institute on Terrestrial Ecosystems (IRET) and the Institute of Polymers, Composites and Biomaterials (IPCB) in Italy, as well as the Technische Universität München (TUM) in Germany.
The findings were published in a paper titled “Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment” in the Journal of Functional Biomaterials.
The primary aim of the research, as articulated by lead researcher Raffaele Conte, was to “formulate an effective drug delivery system using hyaluronic acid hydrogels and chitosan nanoparticles to enhance the therapeutic potential of resveratrol in the treatment of atopic dermatitis.” This objective underscores the team’s commitment to developing innovative approaches to improve patient outcomes in managing this challenging skin condition.
Experimental Process
Primary Technique:
The primary technique employed in this study was the synthesis of chitosan nanoparticles (Res-NPs) through ionotropic gelation, followed by their incorporation into a hyaluronic acid (HA) hydrogel to create a novel drug delivery system for resveratrol aimed at treating atopic dermatitis (AD).
Experiment 1: Synthesis of Chitosan Nanoparticles (Res-NPs)
- Preparation of Chitosan Solution: Chitosan (CS) was solubilized overnight at room temperature in a 1% (v/v) lactic acid solution.
- Preparation of Crosslinking Agent: Sodium tripolyphosphate (TPP) was dissolved to a concentration of 5 mg/mL.
- Loading of Resveratrol: Resveratrol (10 mg) was dissolved in ethanol and gradually added to the CS solution while stirring at 750 rpm for 1 hour to ensure thorough mixing.
- Formation of Nanoparticles: The mixture was then cooled and centrifuged to obtain the Res-NPs, which were washed with distilled water to remove unencapsulated Res.
Data Collection and Analysis: Particle size and zeta potential were measured using the NanoSight NS300 Nanoparticle Tracking Analysis (NTA) system. Encapsulation efficiency (EE) was calculated by comparing the total amount of loaded Res to the amount of free Res detected in the supernatant.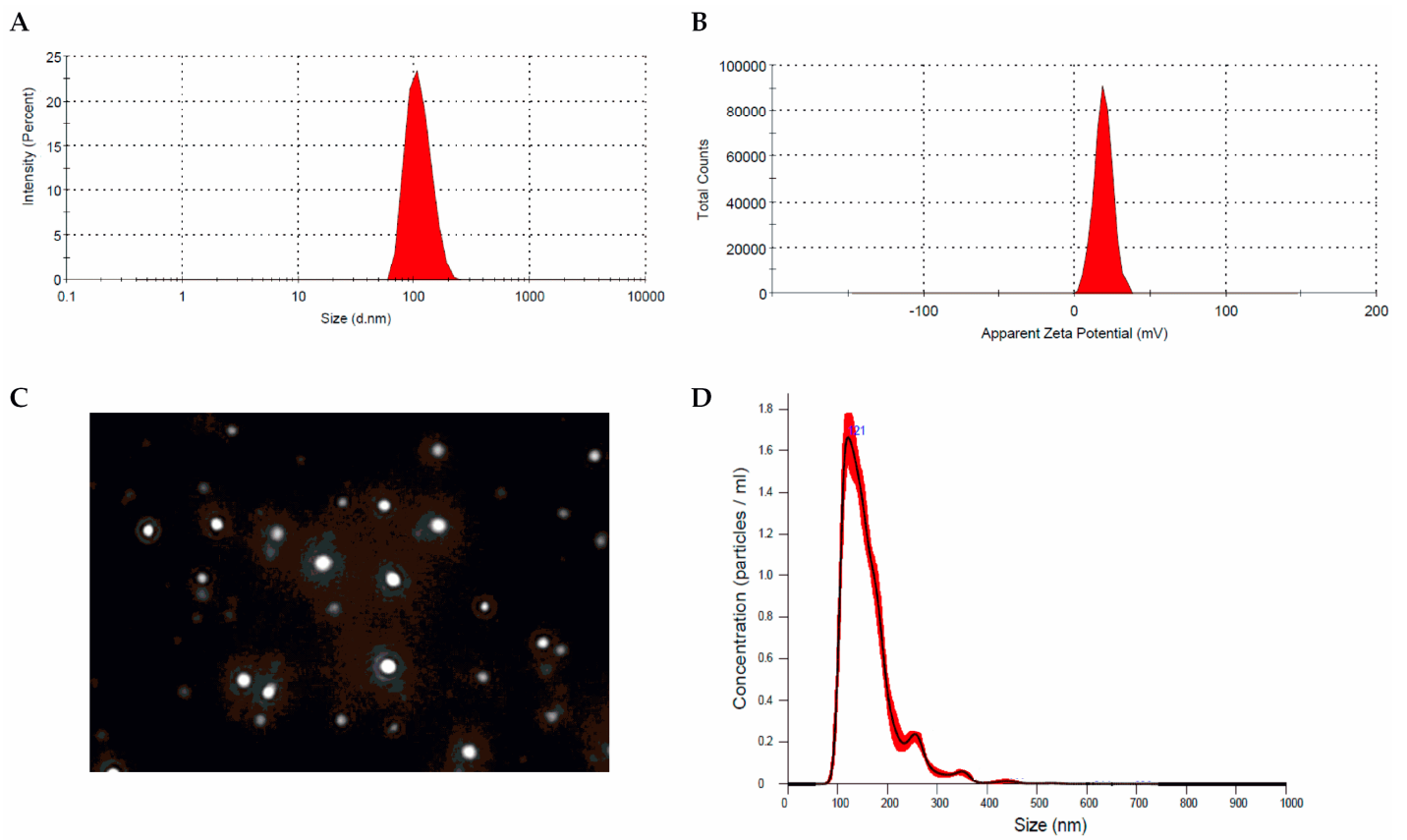
Figure 1. Res-NPs. (A) Size distribution, (B) zeta potential profile, © screenshot of representative NTA video, and (D) NTA measurements for Res-NPs in suspension. Frequency distributions are averages of 3 measurements
Result: The optimal formulation was identified with an average particle size of 121.22 nm, a zeta potential of +19.4 mV, and an EE of 76.18%, indicating successful encapsulation of resveratrol.
Novel Aspects: This experiment introduced a systematic approach to optimize the chitosan-to-TPP ratio, resulting in nanoparticles with high encapsulation efficiency and favorable characteristics for skin delivery, surpassing traditional methods that often yield larger, less stable formulations.
Experiment 2: Preparation of Hyaluronic Acid Hydrogel (Res@gel)
- Hydrogel Preparation: A 4% (w/v) HA solution was prepared by dissolving HA in double-distilled water while stirring for 24 hours.
- Incorporation of Nanoparticles: Lyophilized Res-NPs were added to the HA solution at varying concentrations (1%, 5%, and 10% w/w) and stirred for an hour at 4°C to ensure homogeneity.
- Gelation Process: The solution was then allowed to form a stable gel at room temperature.
Data Collection and Analysis: The hydrogel’s physicochemical properties, such as swelling ratio and rheological behavior, were evaluated. The swelling ratio was calculated by measuring the wet and dry weights of the hydrogels after immersion in saline solution.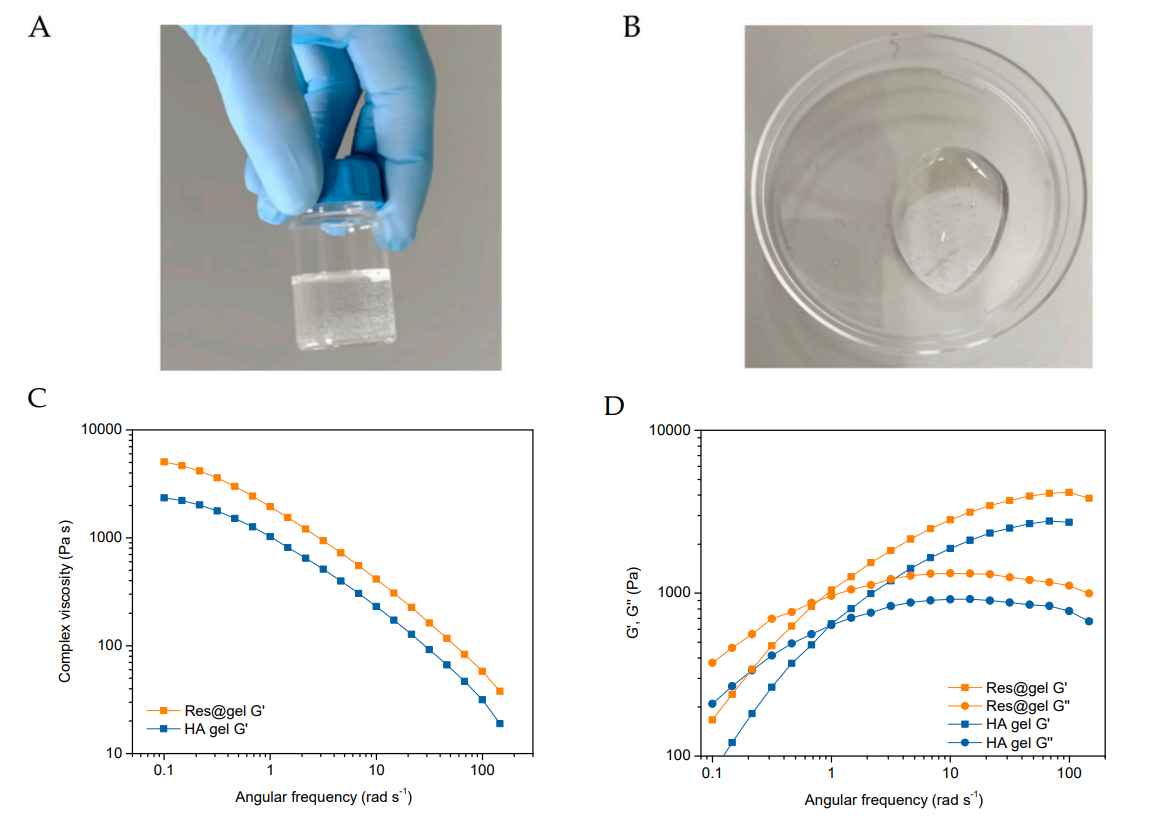
Figure 2. appearance and rheological properties of the HA-based hydrogels. (A,B) Representative images of Res@gel10. Dependence of © viscosity and (D) viscoelastic on the angular frequency of HA and Res@gel10.
Result: The Res@gel displayed significant swelling capacity and viscoelastic properties, indicating that the incorporation of Res-NPs did not adversely affect the hydrogel’s integrity.
Novel Aspects: The integration of Res-NPs into HA hydrogels demonstrated enhanced biocompatibility and controlled release profiles, providing a more effective topical delivery system compared to conventional hydrogels lacking nanoparticle incorporation.
Experiment 3: In Vitro Release and Stability Studies
- Release Testing: A dialysis bag method was utilized to assess the cumulative release of resveratrol from Res@gel over a period of 8 days in phosphate-buffered saline (PBS, pH 7.4) at 37°C.
- Stability Studies: Res-NPs were stored at 4°C and 25°C for 14 days, with measurements taken to monitor changes in size and polydispersity index (PDI).
Data Collection and Analysis: The amount of resveratrol released was quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS), while statistical analysis was performed to evaluate the significance of release profiles.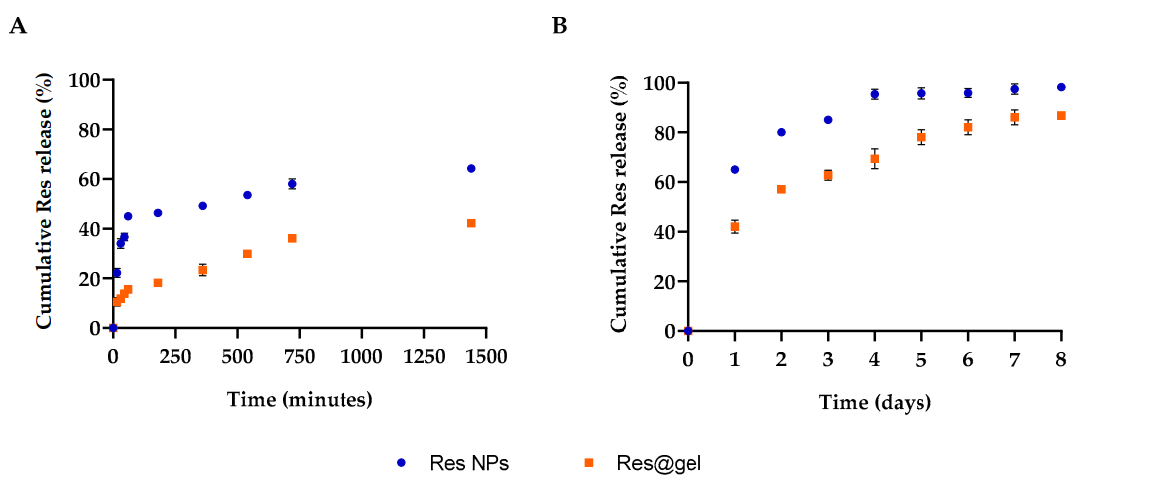
Figure 3. Cumulative Res release from Res NPs and Res@gel10 in phosphate buffer saline (PBS) after (A) 24 h and (B) 8 days. Six different experiment were expressed as the mean of the obtained values mean (+_SD).
Result: Res@gel exhibited a controlled and sustained release profile, with only 15% of Res released in the first hour, effectively maintaining bioavailability over time compared to the rapid release from free Res-NPs.
Novel Aspects: This controlled release mechanism, facilitated by the HA matrix, is a significant advancement over traditional formulations that often lead to burst release, thus providing a more stable therapeutic effect for chronic conditions like AD.
Experiment 4: In Vitro Cytotoxicity and Efficacy Tests
- Cell Culture: Human keratinocyte cell line (HaCaT) was cultured in DMEM supplemented with fetal bovine serum and antibiotics until 80% confluence.
- Treatment Protocol: Cells were pre-treated with varying concentrations of Res@gel for 24 to 96 hours, followed by stimulation with TNF-α/IFN-γ for 24 hours to mimic AD conditions.
- Cytotoxicity Assay: Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay, with fluorescence readings taken at specified time points.
Data Collection and Analysis: Cytokine levels were measured via ELISA, and mRNA expression of inflammatory markers was quantified using real-time quantitative PCR (qRT-PCR).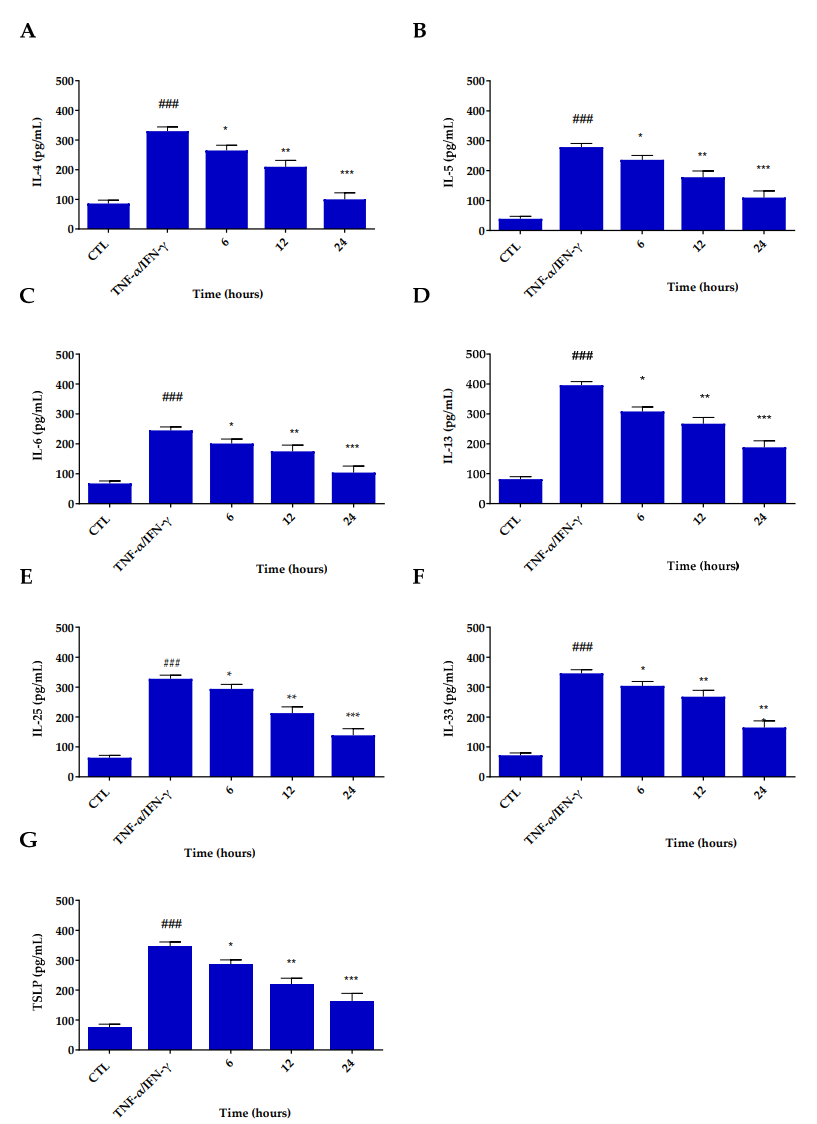
Figure 4. Inhibitory effects of Res@gel10 on inflammatory cytokine secretion in TNF-α/INF-γ-induced HaCaT cells. Secretion of IL-4 (A), IL-5 (B), IL-6 ©, IL-13 (D), IL-25 (E), IL-33 (F), and TSLP (G) was measured by ELISA assay. Cells were pre-treated with Res@gel10 for 24 h, then stimulated with TNF-α/IFN-γ for 24 h. Results are expressed as the mean of three independent experiments ± S.D (n = 3). ### p < 0.001 TNF-α/IFN-γ-treated cells vs. CTL, * p < 0.05, ** p < 0.01, and *** p < 0.001 Res@gel10 vs. TNF-α/IFN-γ-treated cells.
Result: Res@gel demonstrated no cytotoxic effects on keratinocyte proliferation and significantly reduced the secretion of pro-inflammatory cytokines (e.g., IL-4, IL-6, IL-33) compared to the control group.
Novel Aspects: The study established Res@gel as a biocompatible and effective anti-inflammatory agent, overcoming the limitations of traditional treatments by utilizing a natural compound in a targeted delivery system that minimizes side effects.
Conclusion
The successful development of the hyaluronic acid hydrogel containing resveratrol-loaded chitosan nanoparticles (Res@gel) as a topical treatment for atopic dermatitis has been achieved through a systematic and innovative approach. This research effectively combined the biocompatibility and sustained release properties of hyaluronic acid with the anti-inflammatory and antioxidant capabilities of resveratrol, encapsulated within chitosan nanoparticles. The formulation demonstrated favorable physicochemical characteristics, including optimal particle size and encapsulation efficiency, which are critical for enhancing the bioavailability of therapeutic agents.
The highlights of the study include the formulation of a drug delivery system that not only mitigates oxidative stress and inflammation in human keratinocytes but also exhibits no cytotoxic effects on cell proliferation. The Res@gel formulation was shown to significantly reduce the secretion and gene expression of pro-inflammatory cytokines, such as IL-4, IL-6, and IL-33, which are known to be upregulated in atopic dermatitis. These findings suggest that the Res@gel system presents a promising and effective strategy for improving patient outcomes in the management of atopic dermatitis, ultimately aiming to enhance the quality of life for those affected by this chronic skin condition.
Reference
Conte, Raffaele, et al. “Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment.” Journal of Functional Biomaterials, vol. 14, no. 82, 2023, pp. 1-14. MDPI, https://doi.org/10.3390/jfb14020082.
