Editor: Sarah
Cefixime (CFX), a third-generation antibiotic, is commonly used for treating infections such as respiratory, urinary tract, and gonococcal infections. Despite its effectiveness, CFX faces limitations regarding its low solubility and poor intestinal permeability, which significantly reduce its oral bioavailability. These challenges often require the administration of higher doses. To address these issues, recent studies have explored the use of a Self-Emulsifying Drug Delivery System (SEDDS), aiming to enhance the solubility and permeability of CFX. By incorporating CFX into the SEDDS formulation, this study sought to improve its absorption, thereby potentially reducing required dosages and enhancing therapeutic efficacy.
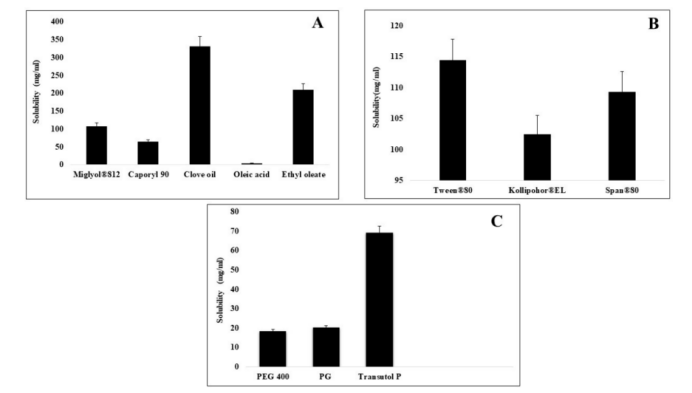
Figure 1: Schematic of Self-Emulsifying Drug Delivery System (SEDDS) for Cefixime.
Contribution and Methodology
Several strategies have been proposed over the years to improve the bioavailability of poorly soluble drugs. These include gastro-retentive systems, nanoparticles, and mucilage-based formulations. However, many of these approaches involve complex and multi-step processes that are not ideal for large-scale industrial manufacturing. This study introduces SEDDS as a simpler, more scalable alternative for enhancing drug solubility and intestinal permeability, with the added benefit of a more straightforward manufacturing process.
The research team used a pseudo-ternary phase diagram to optimize the SEDDS formulation. Excipients were carefully selected based on their ability to enhance drug solubility and emulsification efficiency. The formulations underwent various in vitro tests, including dissolution studies, ex vivo permeability studies, and stability testing, to assess their performance in enhancing CFX absorption.
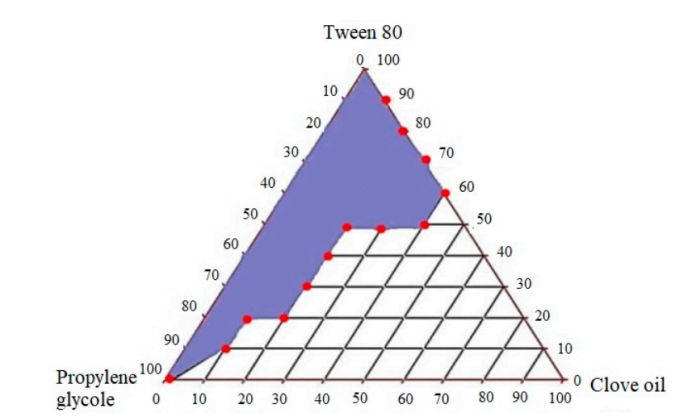
Figure 2: Pseudo-Ternary Phase Diagram for Optimizing SEDDS Formulation.
Key Findings
The SEDDS formulations showed considerable improvements in the solubility and intestinal permeability of CFX. Among the formulations tested, the F-2 formulation demonstrated the highest drug loading (96.32%) and the smallest droplet size (19.01 nm). These characteristics are crucial in enhancing drug absorption, as smaller droplets provide a larger surface area for absorption.
Drug Release Performance: The drug release from the SEDDS formulations was notably better than that of the marketed product. In simulated gastric fluid, up to 97% of CFX was released within 60 minutes, while in simulated intestinal fluid, the release continued for up to 120 minutes. In comparison, the marketed product only released 85% of CFX in the same timeframe. These results suggest that the SEDDS formulations offer more efficient release profiles, improving the bioavailability of CFX.
Permeability Enhancement: Ex vivo studies confirmed a significant increase in the apparent permeability of CFX from the SEDDS formulation. The permeability of the F-2 formulation was 2.7 times higher than that of the marketed product. This increase in permeability is attributed to the smaller droplet size and the solubilization effect provided by the SEDDS formulation, which helps the drug traverse the intestinal membrane more effectively.
Stability: The SEDDS formulations showed promising long-term thermodynamic and chemical stability, with the F-2 formulation demonstrating excellent robustness to dilution and maintaining stability even at different temperatures. The formulation remained stable under accelerated storage conditions, which is a key factor in its potential for industrial-scale production.
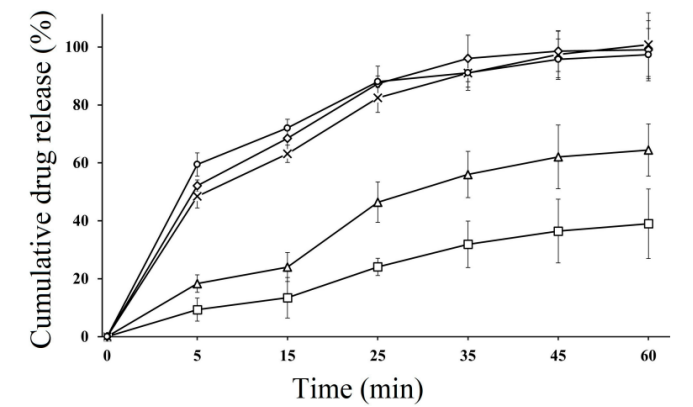
Figure 3: Droplet Size Distribution and Zeta Potential of SEDDS Formulations.
Implications and Applications
The findings from this study have significant implications for the pharmaceutical industry, particularly in improving the bioavailability of poorly soluble antibiotics like cefixime. The enhanced solubility and permeability provided by the SEDDS formulation could lead to more effective treatments, reducing the required dosages and minimizing side effects associated with high drug concentrations. Furthermore, the simplicity and scalability of the SEDDS approach make it an attractive option for industrial production, enabling manufacturers to implement these advancements on a larger scale.
This technology is not limited to cefixime and could be applied to other lipophilic drugs, offering a versatile solution to the common problem of low oral bioavailability. Moreover, by improving the bioavailability of antibiotics like cefixime, this method could contribute to more efficient treatment regimens, helping to mitigate issues such as antibiotic resistance by enabling more effective drug use.
Conclusion
This research highlights the potential of Self-Emulsifying Drug Delivery Systems (SEDDS) to overcome the bioavailability challenges of poorly soluble drugs. By improving both solubility and permeability, the developed SEDDS formulation for cefixime may lead to better therapeutic outcomes. The simplicity and scalability of the SEDDS manufacturing process make it a promising solution for industrial applications, ensuring that these benefits can be realized on a larger scale. This approach not only enhances drug efficacy but also helps reduce the risk of antibiotic resistance, contributing to more effective and sustainable healthcare practices.
Reference
Mahmood, Arshad, et al. “Enhancing Cefixime’s Oral Bioavailability through a Self-Emulsifying Drug Delivery System.” Molecules, vol. 28, no. 6, 2023, p. 2827. MDPI, https://doi.org/10.3390/molecules28062827.
