Editor: Tiffany
A new targeted nanoparticle system, NPF@DOX, has demonstrated enhanced efficacy in treating oral squamous cell carcinoma (OSCC) by combining chemotherapy and photothermal therapy, offering a potential improvement in reducing systemic side effects and enhancing patient outcomes.
Key Highlights
- Research Question:
How can the limitations of current OSCC treatments, such as systemic side effects and low survival rates, be addressed using a targeted drug delivery system? - Research Difficulties:
Developing a nanoparticle system capable of selectively targeting OSCC cells, delivering chemotherapy effectively, and integrating photothermal therapy without causing systemic toxicity. - Key Findings:
NPF@DOX nanoparticles achieved selective drug delivery to OSCC cells, improved cellular uptake, and showed significant antitumor effects in both in vitro and in vivo models when combined with near-infrared (NIR) irradiation. - Innovative Aspects:
The use of FAP-targeted nanoparticles for precise drug delivery and the combination of chemotherapy with photothermal therapy to increase treatment efficacy while minimizing side effects. - Importance of the Study:
This research provides a promising therapeutic strategy for OSCC, with the potential to improve survival rates and quality of life by reducing the adverse effects of conventional treatments.
Challenges in Treating Oral Squamous Cell Carcinoma
Oral squamous cell carcinoma (OSCC) ranks as one of the most common forms of oral cancer, posing a significant global public health challenge due to its high morbidity and mortality rates. This malignancy primarily affects the squamous cells lining the mouth and throat, often leading to severe functional impairments and aesthetic complications. Patients with OSCC frequently experience persistent mouth sores, difficulty swallowing, and unexplained weight loss, symptoms that become more pronounced in advanced stages, where facial defects and speech difficulties further diminish quality of life.
Current treatment modalities for OSCC include surgery, chemotherapy, and radiotherapy. While these approaches aim to eradicate the tumor, they are fraught with limitations. Surgery often results in significant tissue loss and disfigurement, whereas chemotherapy, commonly employing drugs like doxorubicin (DOX), introduces systemic side effects such as cardiotoxicity. Radiotherapy, similarly, can damage surrounding healthy tissues. Despite these interventions, the five-year survival rate for OSCC patients remains low, largely due to tumor recurrence and metastasis, underscoring the need for innovative therapeutic strategies that enhance efficacy while reducing adverse effects.
Developing a Targeted Nanoparticle System for OSCC
A research team from Shanxi Medical University has developed a novel targeted drug delivery system, termed NPF@DOX, designed to improve OSCC treatment outcomes. This system integrates chemotherapy with photothermal therapy (PTT) to deliver DOX selectively to cancer cells while using near-infrared (NIR) light to induce localized hyperthermia, thereby enhancing antitumor effects and minimizing systemic toxicity. The study, published in the International Journal of Nanomedicine in 2023, aimed to create and evaluate this nanoparticle-based platform as a potential advancement over conventional therapies.
The specific objectives of the research were threefold:
- To synthesize and characterize NPF@DOX nanoparticles, which consist of nano-graphene oxide (NGO) functionalized with polyethylene glycol (PEG), fibroblast activation protein (FAP)-targeting peptides, and DOX.
- To assess the nanoparticles’ ability to target FAP, a protein overexpressed in OSCC cells and the surrounding tumor microenvironment, thereby improving drug delivery specificity.
- To evaluate the combined efficacy of chemotherapy and PTT using NPF@DOX in both in vitro and in vivo experimental models.
Evaluating NPF@DOX in Laboratory and Animal Models
The study employed a systematic methodology to synthesize, characterize, and test the NPF@DOX system. Below is an overview of the experimental procedures and key findings:
1. Synthesis and Characterization of NPF@DOX
- The nanoparticles were constructed by functionalizing NGO with PEG to enhance biocompatibility and stability, conjugating FAP-targeting peptides for tumor specificity, and loading DOX as the chemotherapeutic agent.
- Characterization was performed using ultraviolet-visible (UV-Vis) spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), and transmission electron microscopy (TEM). These techniques confirmed the nanoparticles’ structure, revealing a drug-loading efficiency of 44.5 ± 2.1%. Additionally, NPF@DOX exhibited strong photothermal properties, reaching temperatures of approximately 50°C under NIR irradiation, indicating its suitability for PTT.
2. In Vitro Experiments
- Experiment 1: Cellular Uptake
- Procedure: Human OSCC CAL-27 cells were incubated with NPF@DOX, and cellular uptake was visualized using confocal laser scanning microscopy.
- Result: Cells treated with NPF@DOX demonstrated significantly greater DOX accumulation compared to those treated with non-targeted nanoparticles.
- Finding: The FAP-targeting mechanism effectively enhanced the delivery of DOX into OSCC cells, validating the system’s specificity.
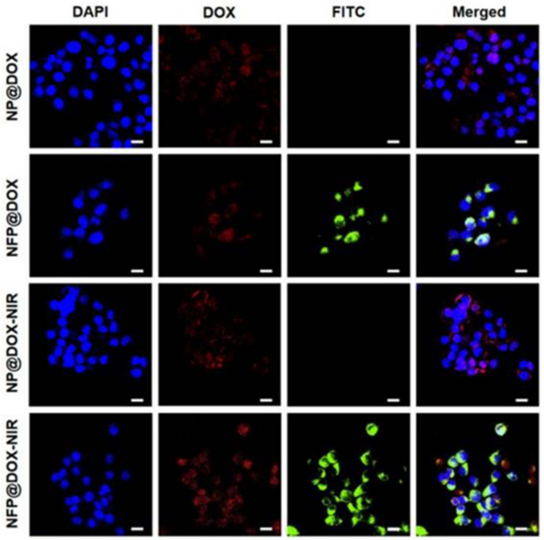
Figure 1. In vitro cellular uptake and drug release. CLSM images of CAL-27 cells treated with NP@DOX or NPF@DOX with or without 808 nm NIR irradiation (1.0 W/ cm2) to evaluate cellular uptake. Scale bar: 20 µm.
- Experiment 2: Cytotoxicity Assay
- Procedure: CAL-27 cells were subjected to various treatments, including NPF@DOX alone, NPF@DOX with NIR irradiation, and control conditions. Cell viability was assessed via a Cell Counting Kit-8 (CCK-8) assay.
- Result: The combination of NPF@DOX and NIR irradiation reduced cell survival to 42%, a markedly lower rate than the 54% observed with non-targeted nanoparticles or other single-modality treatments.
- Finding: The synergy between chemotherapy and PTT significantly outperformed standalone therapies in killing cancer cells.
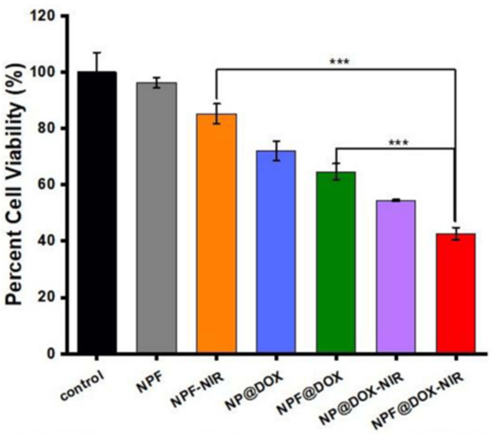
Figure 2. Viability of CAL-27 cells after treatment with control, NPF, NPF-NIR, NP@DOX, NPF@DOX, NP@DOX NIR, and NPF@DOX-NIR.
3. In Vivo Studies
- Experiment 3: Antitumor Effect in Mouse Models
- Procedure: Tumor-bearing mice received intravenous injections of NPF@DOX, followed by NIR irradiation at the tumor site. Tumor volume and systemic toxicity were monitored over 12 days.
- Result: The treatment group exhibited substantial tumor growth inhibition compared to controls, with histological analysis showing no toxicity to major organs, including the heart, liver, spleen, lungs, and kidneys.
- Finding: NPF@DOX, combined with NIR irradiation, effectively targeted and suppressed OSCC tumors in vivo without inducing systemic side effects, highlighting its therapeutic potential.
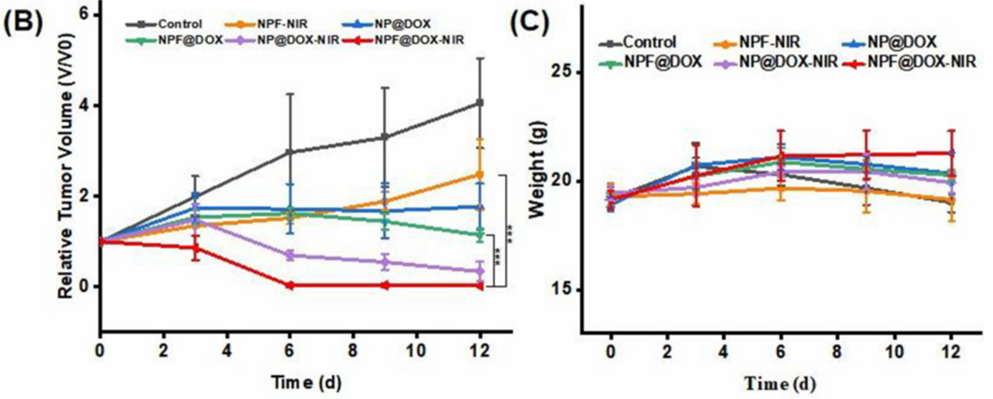
Figure 3. (B) Tumor volume growth after various treatments. (C) Body weight comparisons of mice following intravenous administration of different treatments in tumor-bearing BALB/c nude mice (mean ± SD) with 3 mice in each group.
These results collectively demonstrate that NPF@DOX achieves precise drug delivery, enhances cellular uptake, and amplifies antitumor effects through the integration of chemotherapy and PTT.
Advancing OSCC Treatment with Targeted Dual-Therapy Nanoparticles
The study by the Shanxi Medical University team presents compelling evidence that NPF@DOX represents a significant advancement in OSCC treatment. By targeting FAP, which is overexpressed in both OSCC cells and the tumor microenvironment, the nanoparticles achieved selective drug delivery, reducing the off-target effects commonly associated with conventional chemotherapy. The combination of DOX and NIR-induced hyperthermia yielded superior antitumor outcomes in both cell cultures and mouse models, surpassing the efficacy of standalone chemotherapy or PTT.
Notably, the absence of observable systemic toxicity in vivo addresses a critical limitation of existing OSCC therapies, such as the cardiotoxicity linked to DOX administration. The ability of NPF@DOX to concentrate DOX within tumor cells and release it in a controlled manner under NIR irradiation marks a technological leap forward. These findings suggest that this dual-therapy approach could offer a more effective and less invasive option for OSCC patients, potentially improving survival rates and quality of life.
Reference:
Li, Ran, et al. “A targeted and pH-responsive nano-graphene oxide nanoparticle loaded with doxorubicin for synergetic chemo-photothermal therapy of oral squamous cell carcinoma.” International Journal of Nanomedicine (2023): 3309-3324.
