Editor: Nina
Researchers develop a dissolving microneedle system combined with nanosuspension and co-grinding techniques to enhance the transdermal delivery of ketoprofen, improving its solubility and therapeutic efficacy for osteoarthritis management.
Key Preview
Research Question
The study investigates how to enhance the transdermal delivery of ketoprofen, a non-steroidal anti-inflammatory drug, by utilizing dissolving microneedles (DMN) in combination with nanosuspension (NS) and co-grinding (CG) techniques.
Research Design and Strategy
This research employs a systematic experimental design focusing on the formulation of DMN containing ketoprofen-loaded nanosuspension and co-ground materials. The performance of these formulations is evaluated through various characterization techniques and in vitro permeation studies.
Method
The study formulates ketoprofen nanosuspension using poly(vinyl alcohol) (PVA) as a stabilizer and employs co-grinding with PVA and poly(vinyl pyrrolidone) (PVP). The resultant formulations are then characterized for particle size, zeta potential, and drug release profiles before being assessed for their transdermal delivery capabilities.
Key Results
The most effective DMN formulations were F4-MN-NS (PVA 5%-PVP 10%) and F11-MN-CG (PVA 7.5%-PVP 15%) with cumulative drug permeation amounts of 3.88 ± 0.46 µg and 8.73 ± 1.40 µg after 24 hours, respectively.
Significance of the Research
This research presents a novel approach for improving the transdermal delivery of ketoprofen, potentially leading to better therapeutic outcomes and reduced gastrointestinal side effects associated with oral administration.
Introduction
Inflammation is a complex biological response that serves as the body’s defense mechanism against harmful stimuli such as pathogens, irritants, and damaged cells. One of the most prevalent inflammatory conditions is osteoarthritis, a degenerative joint disease characterized by the breakdown of cartilage, leading to pain, stiffness, and decreased mobility. According to recent health statistics, the prevalence of joint diseases, including osteoarthritis, is significant, affecting millions globally and impacting their quality of life. Effective management of osteoarthritis often relies on non-steroidal anti-inflammatory drugs (NSAIDs), such as ketoprofen, which are widely prescribed to alleviate pain and reduce inflammation.
Traditionally, ketoprofen is administered orally, a common strategy employed in the treatment of inflammatory diseases. While this route of administration is convenient and effective for many medications, it poses several distinct challenges. The oral delivery of ketoprofen can lead to gastrointestinal side effects, including irritation and bleeding, due to its mechanism of action on the gastric mucosa. Furthermore, the first-pass metabolism significantly reduces the bioavailability of the drug, necessitating higher doses that can exacerbate adverse effects. This presents a dilemma for healthcare providers, as they must balance effective pain relief with the potential for harmful side effects.
The limitations of oral administration have prompted researchers to explore alternative drug delivery strategies that can enhance the therapeutic efficacy of NSAIDs while minimizing adverse effects. One innovative approach is the use of dissolving microneedles (DMN) for transdermal drug delivery. This method allows for the pain-free insertion of drug-loaded microneedles into the skin, bypassing the gastrointestinal tract and first-pass metabolism, ultimately improving bioavailability. Additionally, combining DMN technology with advanced formulations such as nanosuspensions and co-grinding techniques offers the potential to further enhance the solubility and permeability of poorly soluble drugs like ketoprofen. This novel strategy aims to provide a more effective and patient-friendly alternative to traditional oral therapies, addressing the challenges of current treatment regimens while improving overall therapeutic outcomes.
Research Team and Aim
The research team is led by Delly Ramadon, who conducted this study in collaboration with Fathin Ulayya, Annisa Sakinah Qur’ani, Iskandarsyah Iskandarsyah, Yahdiana Harahap, Qonita Kurnia Anjani, Vania Aileen, Pietradewi Hartrianti, and Ryan F. Donnelly. The team represents esteemed institutions, including Universitas Indonesia and Queen’s University Belfast. Their paper, titled “Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen,” was published in the journal Pharmaceuticals in March 2023.
The aim of the research, as stated by the lead researcher, was to “formulate and evaluate DMN systems that utilize nanosuspension and co-grinding techniques for enhanced transdermal delivery of ketoprofen, aiming to overcome the drug’s solubility issues.”
Experimental Process
Primary Technique: Dissolving Microneedles (DMN) Fabrication
The primary technique employed in this study was the fabrication of dissolving microneedles (DMN) containing either nanosuspension (NS) or co-ground (CG) forms of ketoprofen. This method capitalizes on the ability of microneedles to penetrate the stratum corneum and deliver drugs transdermally without causing pain, thereby improving the bioavailability of ketoprofen, which is known for its low solubility.
Key Steps of the Experiment: Formulation of Nanosuspension
- Preparation of Nanosuspension: Ketoprofen was combined with poly(vinyl alcohol) (PVA) in varying concentrations (0.5%, 1%, and 2%). The mixture was stirred until homogeneous.
- Ultrasonication: The homogeneous suspension was subjected to ultrasonication for 5 minutes at a pulse rate of 10 seconds on and 5 seconds off, with an amplitude set at 80% to reduce particle size and form the nanosuspension.
- Characterization: The resulting nanosuspension was characterized for particle size and zeta potential using a particle size analyzer, ensuring that the formulations had a particle size below 1000 nm and an appropriate zeta potential for stability.
Data Collection and Analysis:
Data were collected through dynamic light scattering (DLS) to determine the particle size distribution and zeta potential. Statistical analysis was performed using ANOVA to compare the effects of varying PVA concentrations on particle size, with results expressed as mean ± SD.
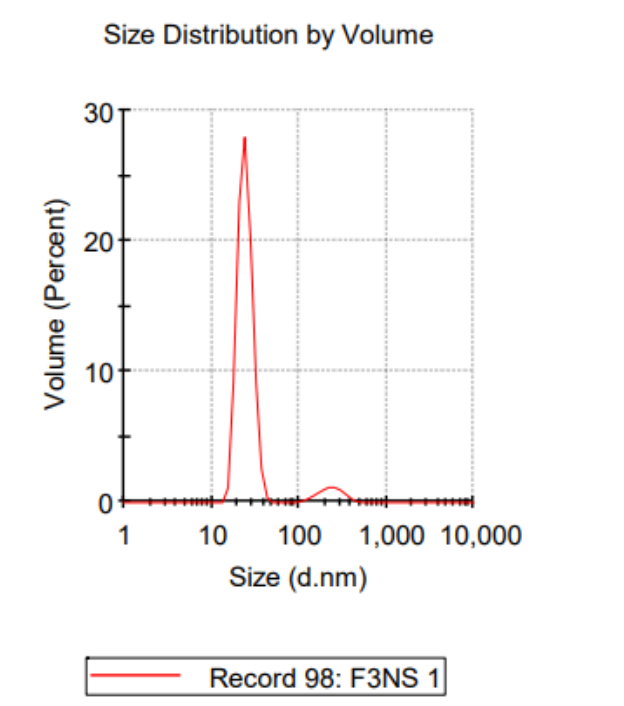
Figure 1. Representative of DLS graph of NS-3.
Results:
The nanosuspension with 2% PVA (NS-3) exhibited the smallest particle size, averaging 124.3 ± 26.15 nm, and a zeta potential of -15.7 ± 3.72 mV, indicating good stability. This demonstrated that increasing the concentration of PVA effectively reduced the particle size and improved the stability of the nanosuspension.
Key Steps of the Experiment: Co-Grinding of Ketoprofen
- Preparation of Co-Grinded Mixtures: Different weight ratios of ketoprofen with PVA and poly(vinyl pyrrolidone) (PVP) were ground using a mortar and pestle for 60 minutes to form a homogeneous mixture.
- Characterization: The co-ground samples were analyzed using Fourier Transform Infrared Spectroscopy (FTIR) and X-ray diffraction (XRD) to assess the changes in crystallinity and potential interactions between ketoprofen and the polymers.
Data Collection and Analysis:
FTIR spectra were analyzed to identify any potential interactions between ketoprofen and the polymers, while XRD patterns provided insight into the crystallinity of the co-ground formulations.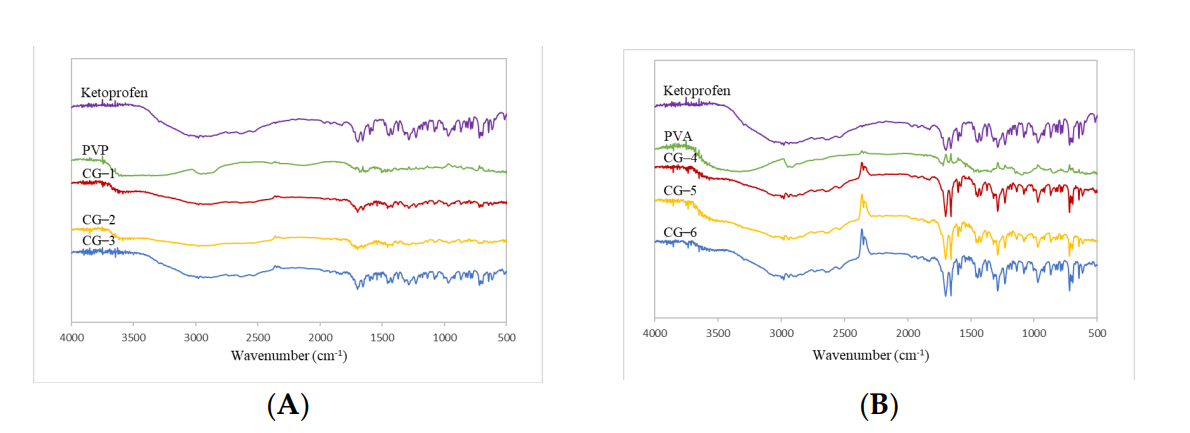
Figure 2. Co−grinded Ketoprofen FTIR Spectrum with PVP and (B) Co−grinded Ketoprofen FTIR Spectrum with PVA.
Results:
FTIR analysis revealed no significant spectral changes in the co-ground formulations compared to pure ketoprofen and PVA or PVP, indicating a lack of chemical interaction. XRD results demonstrated a reduction in crystallinity, suggesting enhanced solubility through co-grinding.
Key Steps of the Experiment: Fabrication of DMN
- Micromolding Technique: The selected formulations of nanosuspension and co-ground ketoprofen were cast into a silicone micromold designed with microneedle arrays.
- Drying Process: The assembled molds were subjected to a positive air pressure of 3-4 bar for 15 minutes to ensure proper filling, followed by a drying period of 48 hours at 30 °C to allow the microneedles to form.
Data Collection and Analysis:
The physical properties of the microneedles were evaluated using a digital microscope and scanning electron microscopy (SEM) to assess morphology and structural integrity.
Figure 3. EM Observations for (A) Ketoprofen, (B) PVP, © PVA, (D) CG-1, (E) CG-2, (F) CG-3, (G) CG-4, (H) CG-5, and (I) CG-6 at magnification of 500× (scale bar shown in the figure: 50 µm).
Results:
The DMN formulations exhibited sharp, well-defined needle structures, with successful formation confirmed by the absence of brittle failures. The mechanical strength tests indicated that formulations containing a combination of PVA and PVP demonstrated superior mechanical properties.
Key Steps of the Experiment: In Vitro Permeation Studies
- Preparation of Franz Diffusion Cells: The in vitro permeation study was conducted using Franz diffusion cells, with rat skin mounted between the donor and receptor compartments.
- Application of DMN: Each DMN formulation was applied to the skin, and samples were collected at predetermined time intervals for up to 24 hours to assess drug permeation.
Data Collection and Analysis:
Cumulative amounts of ketoprofen permeated into the receptor compartment were measured using HPLC, allowing for a precise quantification of drug delivery.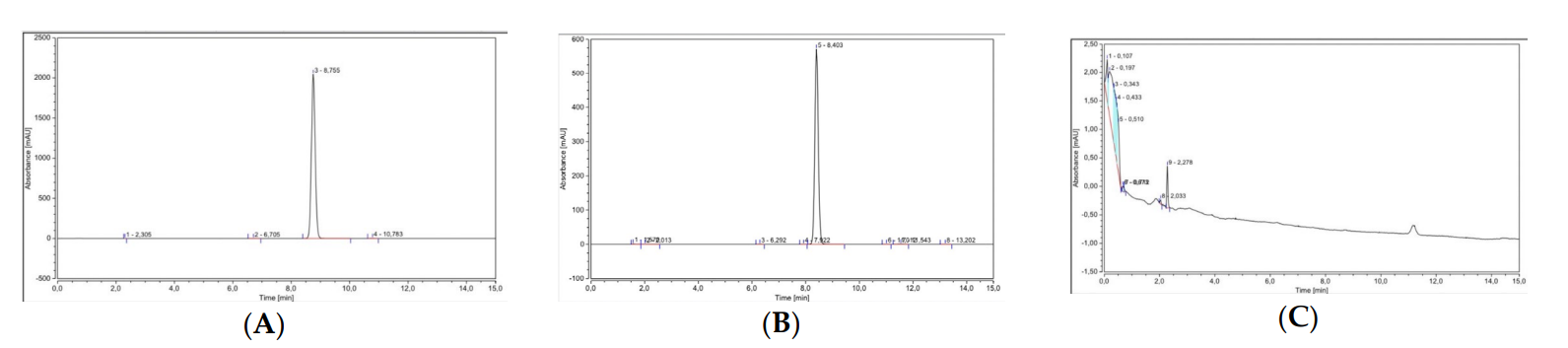
Figure 4. Chromatogram and spectrum of ketoprofen as determined by HPLC. (A)Ketoprofen standard. (B)Ketoprofen-PVA mixture ©Blank sample.
Results:
The cumulative drug permeation for the DMN formulations containing CG ketoprofen significantly outperformed those with NS, with F11-MN CG achieving 8.73 ± 1.40 µg after 24 hours, indicating a higher bioavailability and effective transdermal delivery.
Novel Aspects and Advantages:
This study introduces the innovative use of combining DMN with nanosuspension and co-grinding techniques for enhancing the transdermal delivery of ketoprofen. Unlike traditional nano-delivery systems, which often face challenges related to solubility and bioavailability, the methods developed here significantly improve drug release rates and therapeutic efficacy. The integration of these techniques not only enhances the solubility of poorly soluble drugs but also provides a more patient-friendly alternative to traditional delivery methods, reducing gastrointestinal side effects associated with oral administration.
Conclusion
The successful development of the dissolving microneedle (DMN) system for the transdermal delivery of ketoprofen was achieved through a systematic approach that combined innovative formulation techniques, namely nanosuspension and co-grinding. These methods significantly enhanced the solubility and permeability of ketoprofen, a drug known for its low bioavailability when administered orally.
This study highlights the effective formulation of DMN systems, demonstrating that the most promising formulations, F4-MN-NS and F11-MN-CG, achieved cumulative drug permeation amounts of 3.88 ± 0.46 µg and 8.73 ± 1.40 µg after 24 hours, respectively. These results underscore the potential of the combined DMN approach to improve therapeutic outcomes for patients with osteoarthritis while minimizing the gastrointestinal side effects associated with conventional oral administration. Overall, the findings from this research offer a promising strategy for enhancing the delivery of poorly soluble drugs via transdermal routes, paving the way for future developments in drug delivery systems.
Reference
Ramadon, Delly, et al. “Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen.” Pharmaceuticals, vol. 16, no. 3, 2023, article 378. MDPI, https://doi.org/10.3390/ph16030378
