Editor: Tiffany
Researchers have developed a novel drug delivery system that enables the sustained and targeted release of itaconate, a cell-impermeable molecule, directly into joint cells, showing significant promise in reducing inflammation and slowing osteoarthritis progression in preclinical models.
Key Highlights
- Research Question:
Can a new drug delivery system improve the therapeutic efficacy of itaconate for treating osteoarthritis? - Research Difficulties:
The clinical translation of itaconate is hindered by its short joint residence time, inefficient delivery, and inability to penetrate cell membranes. - Key Findings:
The study found that itaconate-encapsulated hydrogel microspheres exhibited superior anti-inflammatory and antioxidant effects, outperforming conventional drug delivery methods. - Innovative Aspects:
The use of metal-organic framework-anchored hydrogel microspheres allows for sustained and targeted release of itaconate in a pH-responsive manner. - Importance of the Study:
This research provides a novel strategy to enhance the therapeutic potential of itaconate in osteoarthritis, addressing a critical gap in current treatment options.
Osteoarthritis: A Growing Challenge in Joint Health
Osteoarthritis (OA) is a widespread joint disorder affecting millions globally, characterized by the progressive degeneration of articular cartilage—the protective tissue that cushions the ends of bones in joints. This condition leads to chronic pain, stiffness, and reduced mobility, predominantly impacting weight-bearing joints such as the knees and hips. The paper highlights that OA arises from the breakdown of cartilage, driven by inflammation and oxidative stress within the joint environment. These processes cause cartilage cells, known as chondrocytes, to deteriorate, exacerbating joint damage over time.
Current treatments for OA primarily involve non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics, which alleviate pain and inflammation but fail to address the underlying disease progression. The paper notes that these therapies provide only symptomatic relief and are limited by potential side effects, such as gastrointestinal complications and cardiovascular risks with prolonged NSAID use. Additionally, these treatments do not effectively halt cartilage degradation or repair joint tissue, underscoring the need for innovative therapies that target the root causes of OA, including inflammation and oxidative damage.
Targeting Inflammation and Oxidative Stress in OA Therapy
The study, published in Pharmaceutics in 2023 by researchers from Jiaxing University, Zhejiang Chinese Medical University, and Bengbu Medical College, explores a novel approach to OA treatment using itaconate—a molecule known for its anti-inflammatory and antioxidant properties. However, itaconate’s therapeutic potential is hindered by its inability to penetrate cell membranes and reach intracellular targets effectively. To overcome this barrier, the research team, led by Han Yu, Peng Ren, and colleagues, developed a sophisticated drug delivery system designed to transport itaconate into cells and sustain its release within the joint.
The primary aim of the study was to create and evaluate a delivery system combining itaconate-loaded nanoparticles with hydrogel microspheres. The objectives included assessing the system’s ability to reduce inflammation and oxidative stress in cell-based experiments (in vitro) and to mitigate OA progression in an animal model (in vivo). By addressing the delivery challenges, the researchers sought to enhance itaconate’s efficacy as a disease-modifying treatment for OA.
Developing and Testing a Novel Itaconate Delivery System
Experimental Procedures
- Synthesis of IA-ZIF-8 nanoparticles: Itaconate (IA) was encapsulated into zeolitic imidazolate framework-8 (ZIF-8) nanoparticles via a self-assembly process using zinc ions, 2-methylimidazole, and IA.
- Fabrication of IA-ZIF-8@HMs: IA-ZIF-8 nanoparticles were integrated into gelatin methacrylate (GelMA) hydrogel microspheres through one-step microfluidic technology, followed by UV-light-induced photo-crosslinking.
- In vitro evaluations: Anti-inflammatory and anti-oxidative effects of IA-ZIF-8@HMs were tested on chondrocytes, assessing inflammatory cytokine levels and cell viability under oxidative stress.
- In vivo evaluations: Therapeutic effects of IA-ZIF-8@HMs were evaluated in a monoiodoacetic acid (MIA)-induced osteoarthritis (OA) rat model, using X-ray imaging and histological analysis.
Key Experiments
- In vitro anti-inflammatory efficacy
- Procedure: Chondrocytes were seeded in a 6-well plate and stimulated with interleukin-1β (IL-1β, 10 ng/mL) to trigger inflammation. Cells were then treated with IA-ZIF-8@HMs, and after incubation, levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in the supernatants were quantified using enzyme-linked immunosorbent assay (ELISA) kits.
- Result: Compared to the IL-1β-stimulated control group, the IA-ZIF-8@HM-treated group exhibited a ~50% reduction in TNF-α and a ~45% reduction in IL-6 levels.
- Finding: IA-ZIF-8@HMs effectively reduced inflammation in chondrocytes, showcasing its potential to deliver itaconate and suppress inflammatory responses.
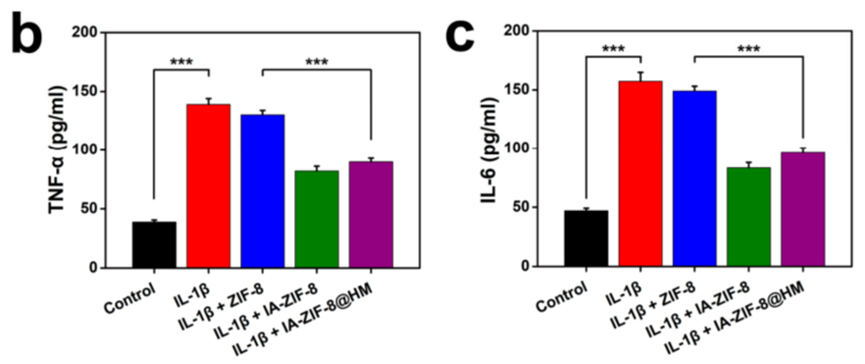
Figure 1. ELISA analysis of TNF-α and IL-6 concentration in cell supernatants.
- In vitro anti-oxidative stress efficacy
- Procedure: Chondrocytes were seeded in a 96-well plate, pretreated with IA-ZIF-8@HMs for 4 hours, and then exposed to hydrogen peroxide (H₂O₂, 300 μM) for 36 hours to induce oxidative stress. Cell viability was measured using the MTT assay.
- Result: Cell viability dropped to ~50% in the H₂O₂-only group, while the IA-ZIF-8@HM-treated group maintained ~80% viability compared to the untreated control.
- Finding: IA-ZIF-8@HMs significantly protected chondrocytes from oxidative stress, enhancing cell survival in conditions simulating OA-related oxidative damage.
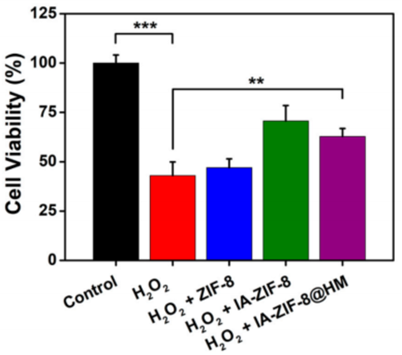
Figure 2. The results of cell viability about chondrocyte co-cultured with H2O2 , H2O2 + ZIF-8, H2O2 + IA-ZIF-8, and H2O2 + IA-ZIF-8@HM (** p < 0.01, *** p < 0.001).
- In vivo therapeutic efficacy in OA model
- Procedure: Male Sprague-Dawley rats received an MIA injection (2.5 mg) in the knee to induce OA and were divided into groups receiving weekly intra-articular injections of IA-ZIF-8@HMs, IA-ZIF-8 nanoparticles, blank hydrogel microspheres, or phosphate-buffered saline (PBS) for 5 weeks. Post-treatment, articular space width was assessed via X-ray imaging, and joint tissues were analyzed histologically with hematoxylin-eosin (H&E) and safranin O-fast green staining.
- Result: The IA-ZIF-8@HM group retained ~85% of the healthy articular space width (vs. 60% in the PBS group). Histology showed reduced cartilage erosion, with an OARSI score of 3.5 (vs. 7.0 for PBS).
- Finding: IA-ZIF-8@HMs outperformed other treatments in preserving joint structure and slowing OA progression, underscoring the efficacy of its sustained-release mechanism.
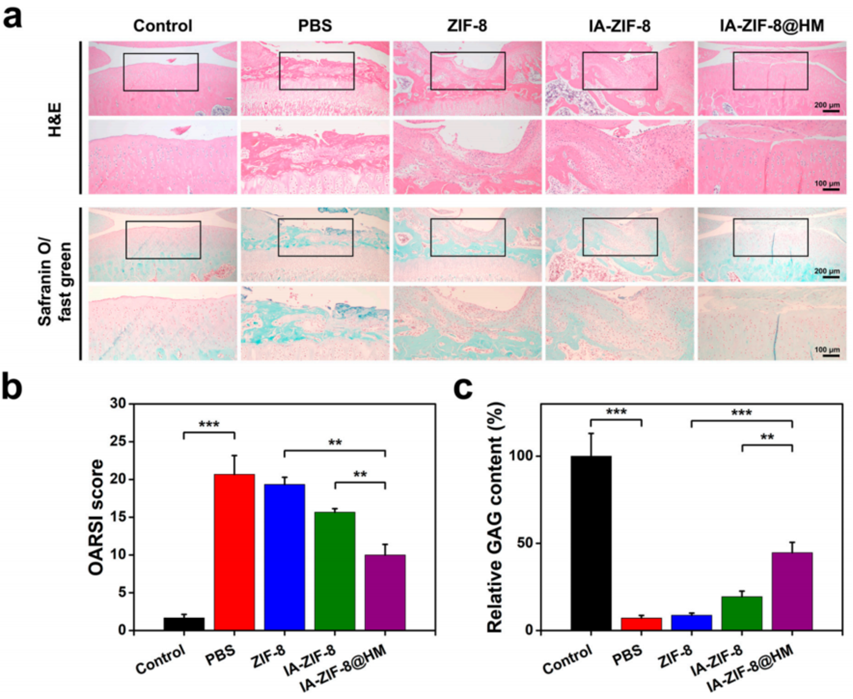
Figure 3. IA-ZIF-8@HM relieved the progression of OA in vivo experiments.
A Step Forward in Osteoarthritis Treatment
The study demonstrates that the IA-ZIF-8@HM delivery system effectively transports itaconate into cells, leveraging the pH-responsive properties of nanoparticles and the sustained-release capabilities of hydrogel microspheres. In cell experiments, this system markedly reduced inflammation and oxidative stress, key contributors to OA progression. In rats, weekly injections led to improved joint structure and reduced cartilage degradation compared to controls, highlighting its potential as a disease-modifying therapy.
The dual-structured approach addresses critical limitations of current OA treatments by delivering a therapeutic agent directly to the site of action and maintaining its presence over time. The use of microfluidics ensures the production of uniform microspheres, enhancing the system’s reproducibility and clinical feasibility. The researchers emphasize the system’s biocompatibility and efficacy, positioning it as a promising advancement in the field of joint disease treatment.
Reference:
Yu, Han, et al. “Intracellular delivery of itaconate by metal–organic framework-anchored hydrogel microspheres for osteoarthritis therapy.” Pharmaceutics 15.3 (2023): 724.
