Editor: Nina
Scientists develop a solid lipid nanoparticle system for enhanced delivery and sustained release of 5-fluorouracil, significantly improving its cytotoxicity against skin melanoma and squamous cell carcinoma while ensuring safety and biocompatibility.
Key Preview
- Research Question
The study investigates how solid lipid-based nanoparticles can enhance the delivery and efficacy of 5-fluorouracil (5-FU) in treating skin melanoma and squamous cell carcinoma. - Research Design and Strategy
The research utilized a hot melt encapsulation technique to prepare solid lipid nanoparticles (SLNs) and analyzed their physicochemical properties, drug release behavior, cytotoxicity, and permeation. - Method
5-FU-loaded SLNs were prepared using Precirol® ATO 5 as the solid lipid and Poloxamer 188 with Tween 80 as surfactants. Key assessments included particle size, zeta potential, entrapment efficiency, and in-vitro cytotoxicity tests. - Key Results
The SLNs demonstrated a particle size range of 76.82 to 327 nm with an entrapment efficiency of 63.46% to 76.08%. In vitro studies showed enhanced cytotoxicity against melanoma and squamous cell carcinoma cells compared to free 5-FU. - Significance of the Research
This study signifies a potential breakthrough in topical cancer therapies by using SLNs to improve drug delivery, enhance therapeutic effects, and reduce side effects associated with conventional chemotherapy.
Introduction
Skin cancer is one of the most prevalent forms of cancer globally, affecting millions of individuals each year. Among the various types of skin cancer, melanoma and squamous cell carcinoma (SCC) are particularly concerning due to their aggressive nature and potential for metastasis. Melanoma arises from melanocytes, the pigment-producing cells of the skin, while SCC originates from keratinocytes, the predominant cell type in the outer layer of the skin. The increasing incidence of skin cancer is often attributed to excessive sun exposure, indoor tanning, and other environmental factors that damage the skin’s cellular structure.
Traditional treatment strategies for skin cancer typically involve surgical excision, radiation therapy, and systemic chemotherapy. Among these, chemotherapy using agents like 5-fluorouracil (5-FU) has gained prominence, particularly for superficial lesions and non-invasive cases. The standard approach to delivering 5-FU often involves topical applications or systemic administration, aiming to achieve localized therapeutic concentrations at the tumor site.
However, current drug delivery methods face significant challenges, primarily due to the hydrophilic nature of 5-FU, which limits its permeability through the skin barrier. This low skin permeability often results in suboptimal drug concentrations at the target site, leading to reduced therapeutic efficacy and increased incidence of side effects. Moreover, the rapid clearance of 5-FU from the circulation can necessitate higher dosing frequencies, further complicating treatment regimens and potentially increasing patient discomfort.
To address these challenges, innovative drug delivery strategies have emerged, focusing on enhancing the stability, solubility, and absorption of therapeutic agents. One such approach is the development of solid lipid nanoparticles (SLNs), which offer a promising solution for improving the delivery and efficacy of 5-FU. By encapsulating the drug within lipid matrices, SLNs can enhance drug solubility, prolong release times, and improve skin penetration capabilities. This innovative delivery system not only aims to maximize the local therapeutic effect but also minimizes systemic side effects, representing a significant advancement in the management of skin melanoma and SCC.
Research Team and Aim
The research team was led by Asadullah Madni, who, along with co-authors Ahsan Ali, Hassan Shah, Talha Jamshaid, Nasrullah Jan, Safiullah Khan, Muhammad Muzamil Khan, and Muhammad Ahmad Mahmood, conducted the study at The Islamia University of Bahawalpur, Pakistan. This research was carried out in 2023 and was published in the journal PLoS ONE under the title “Solid lipid-based nanoparticulate system for sustained release and enhanced in-vitro cytotoxic effect of 5-fluorouracil on skin Melanoma and squamous cell carcinoma.”
The aim of the research, as stated by the lead researcher, was to develop a solid lipid nanoparticle (SLN) system that could effectively encapsulate 5-fluorouracil (5-FU), thereby improving its delivery to skin cancer sites, enhancing its cytotoxic effects, and ensuring safety and biocompatibility for potential therapeutic applications.
Experimental Process
Experiment 1: Preparation of 5-FU-Loaded Solid Lipid Nanoparticles (SLNs)
Primary Technique: The primary technique used in this study was the hot melt encapsulation (HME) method, chosen for its ability to avoid toxic organic solvents and facilitate the incorporation of hydrophilic drugs like 5-fluorouracil (5-FU) into a lipid matrix.
Key Steps: The SLNs were prepared by melting Precirol® ATO 5 (100 mg, 75 mg, or 50 mg) at 5°C above its melting point (approximately 70°C) in a glass vial. 5-FU (5 mg) was added to the melted lipid, followed by 0.5 mL ethanol to ensure homogenous mixing, with stirring at 500 rpm for 5 minutes using a magnetic stirrer. Concurrently, an aqueous phase (5 mL) containing Poloxamer 188 (1–3% w/v) and Tween 80 (0.5% w/v) was heated to the same temperature. The lipid phase was added dropwise to the aqueous phase under continuous stirring at 1000 rpm. The mixture was homogenized at 12,000 rpm for 3 minutes using a Polytron PT 1200E homogenizer, cooled to room temperature, sonicated for 5 minutes (40 kHz), and centrifuged at 14,000 rpm for 20 minutes. The resulting SLNs were lyophilized at -45°C under reduced pressure for 24 hours.
Data Collection and Analysis: Particle size, zeta potential, and polydispersity index (PDI) were measured using dynamic light scattering (DLS) with a Zeta Sizer-ZS90 at 25°C and a 90° angle, with samples analyzed in triplicate. Entrapment efficiency (%EE) was determined indirectly by centrifuging SLNs at 12,000 rpm, measuring unentrapped 5-FU in the supernatant via UV-visible spectrophotometry at 266 nm, and calculating %EE using the formula: %EE = [(Total 5-FU – Free 5-FU) / Total 5-FU] × 100.
Result: The SLNs exhibited particle sizes ranging from 76.82 ± 1.48 nm to 327 ± 4.46 nm, zeta potentials from -11.3 ± 2.11 mV to -28.4 ± 2.40 mV, PDI below 0.5, and %EE between 63.46 ± 1.13% and 76.08 ± 2.42%, with SLN4 (100 mg Precirol, 1% Poloxamer) showing optimal characteristics (100.3 ± 2.86 nm, -28.4 ± 2.40 mV, 76.08 ± 2.42% EE).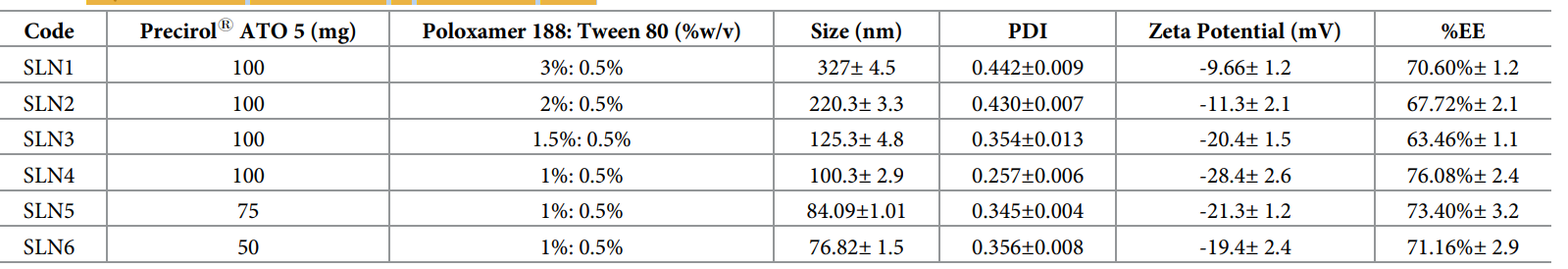
Table 1. Physicochemical characteristics of 5-FU-loaded SLNs.
Novel Aspects and Advantages: The HME method’s solvent-free approach enhances safety and scalability compared to traditional solvent-based nano-delivery systems. The use of Precirol® ATO 5, with its loose fatty acid structure, improves hydrophilic drug entrapment, addressing the limitations of conventional SLNs that favor lipophilic drugs.
Experiment 2: In-Vitro Drug Release Study
Primary Technique: The dialysis bag diffusion technique was employed to assess the release profile of 5-FU from SLNs, selected for its ability to mimic physiological conditions and maintain sink conditions.
Key Steps: Lyophilized 5-FU-loaded SLNs (equivalent to 5 mg 5-FU) were dispersed in 3 mL phosphate buffer saline (PBS, pH 7.4) and placed in dialysis bags (MWCO: 10 kDa), pre-soaked in distilled water for 12 hours. The bags were sealed and immersed in 200 mL PBS (pH 7.4) in a USP Type II dissolution apparatus at 37 ± 0.5°C and 50 rpm. Samples (2 mL) were withdrawn at 0.5, 1, 2, 3, 6, 12, 24, and 48 hours, replaced with fresh PBS, and analyzed for 5-FU content using UV-visible spectrophotometry at 266 nm.
Data Collection and Analysis: Drug release percentages were calculated from absorbance readings, plotted against time, and fitted to kinetic models (Zero Order, First Order, Higuchi, Korsmeyer-Peppas) using DD Solver in MS Excel. Regression coefficients (R²) and release exponent (n) were determined to elucidate release mechanisms.
Result: 5-FU release from SLNs showed a biphasic pattern: an initial burst (40–45% within 3 hours) followed by sustained release (88–98% over 48 hours), compared to complete release of free 5-FU within 6 hours. The Korsmeyer-Peppas model best fit the data (R² = 0.9789–0.9860), with n values (0.276–0.292) indicating Fickian diffusion.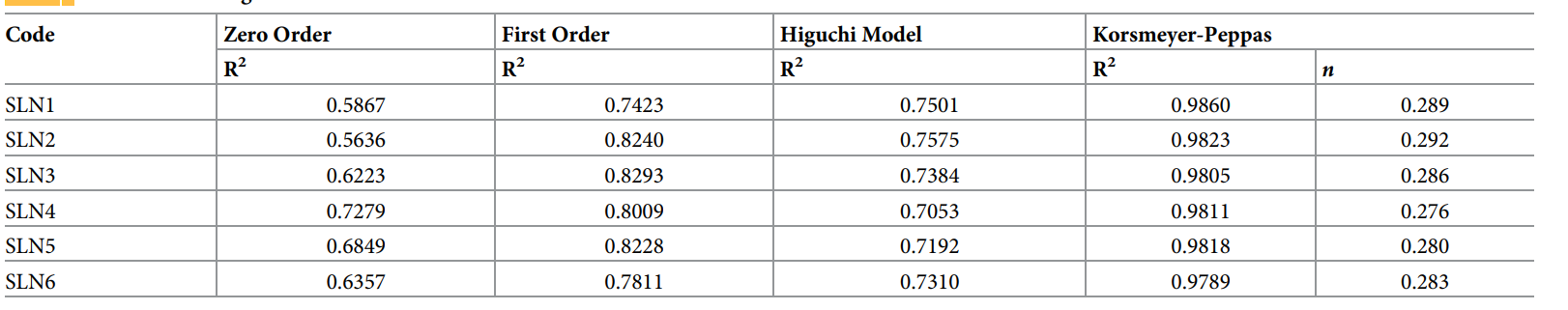
Table 2. Kinetic modeling of 5-FU-loaded SLNs.
Novel Aspects and Advantages: The biphasic release profile enhances initial therapeutic levels and sustains delivery, reducing dosing frequency compared to traditional systems lacking controlled release.
Experiment 3: In-Vitro Cytotoxicity Study
Primary Technique: The Cell Titer-Blue® assay was used to evaluate cytotoxicity against B16F10 (murine melanoma) and A-431 (human squamous cell carcinoma) cell lines, chosen for its sensitivity in measuring cell viability.
Key Steps: Cells were cultured in DMEM with 10% FBS and antibiotics, seeded at 7,000 cells/well in 96-well plates, and incubated at 37°C with 5% CO₂ for 24 hours. Four groups were tested: control (untreated), blank SLNs, 5-FU solution (12.5–400 µM), and 5-FU-loaded SLNs (12.5–400 µM 5-FU equivalent). After 24 or 48 hours of treatment, 20 µL Cell Titer-Blue® reagent was added, incubated for 4 hours, and fluorescence was measured at 560/590 nm using a BioTek plate reader.
Data Collection and Analysis: Cell viability percentages were calculated relative to controls, with data from triplicate experiments analyzed via ANOVA and Tukey’s post hoc test (GraphPad Prism 8) to determine statistical significance (p < 0.05).
Result: 5-FU-loaded SLNs exhibited significantly higher cytotoxicity (e.g., ~20–30% lower viability) than free 5-FU at all concentrations after 48 hours (p < 0.05), while blank SLNs showed negligible effects (>95% viability), confirming biocompatibility.
Novel Aspects and Advantages: Enhanced cytotoxicity stems from improved cellular uptake and sustained release, outperforming traditional 5-FU formulations with lower efficacy due to rapid clearance.
Experiment 4: Cellular Uptake Studies
Primary Technique: Flow cytometry and fluorescence microscopy were combined to quantify and visualize SLN uptake, selected for their precision in assessing nanoparticle internalization.
Key Steps: For flow cytometry, B16F10 and A-431 cells (1 million/well) were seeded in 6-well plates, incubated for 24 hours, and treated with Rhodamine-PE (Rh-PE, 1 mol%)-loaded SLNs for 4 hours. Cells were trypsinized, washed thrice with PBS (pH 7.4), centrifuged at 1000 rpm for 5 minutes, and resuspended in 200 µL PBS. Fluorescence was measured at 488 nm excitation and 530/30 nm emission, collecting 10,000 events. For microscopy, 50,000 cells/well were seeded on cover glass in 24-well plates, treated with Rh-PE-loaded SLNs for 4 hours, fixed with 4% paraformaldehyde, stained with Hoechst 33342 (10 µg/mL), and imaged using a KEYENCE BZ-X710 microscope.
Data Collection and Analysis: Flow cytometry data quantified uptake as fold-increase over control, analyzed with ANOVA (p < 0.0001). Microscopy images visually confirmed uptake, with red (Rh-PE) and blue (Hoechst) fluorescence indicating SLN localization.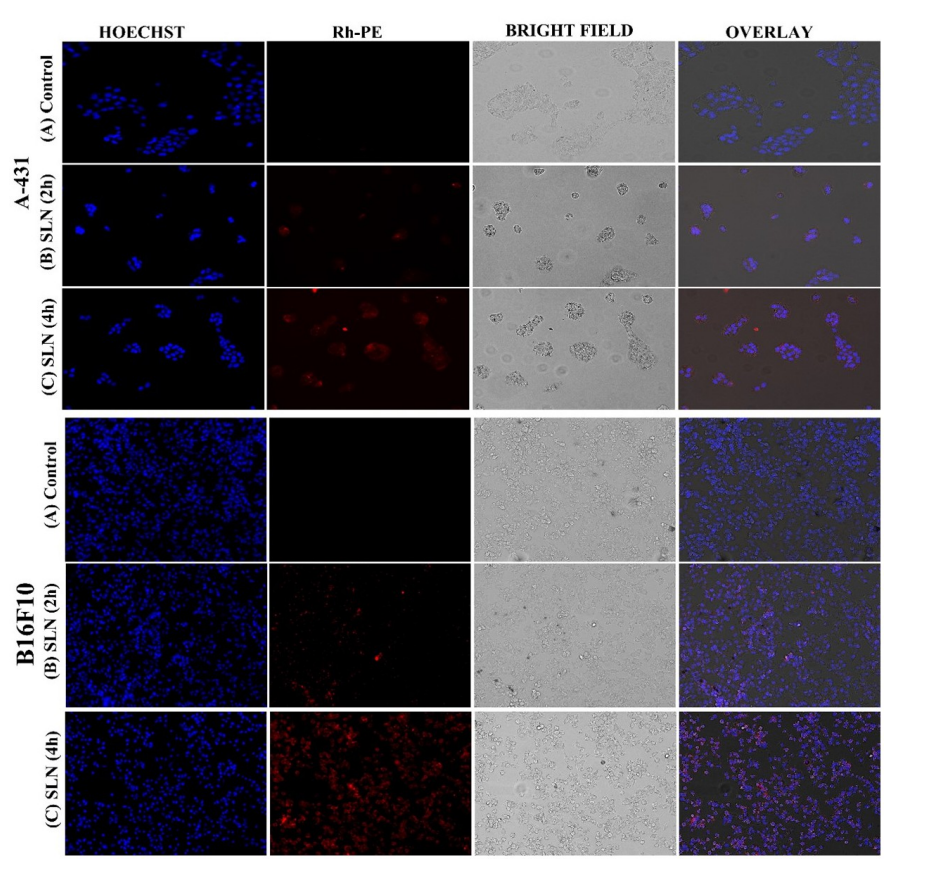
Figure 1. Qualitative cellular uptake studies of control vs treated cells with SLNs.
Result: SLNs showed 12.66-fold (B16F10) and 15.63-fold (A-431) higher uptake than controls after 4 hours (p < 0.0001), with microscopy revealing time-dependent internalization.
Novel Aspects and Advantages: Dual-technique validation and high uptake efficiency highlight SLNs’ superiority over traditional systems with poor cellular penetration.
Experiment 5: Ex-Vivo Permeation and Skin Retention Studies
Primary Technique: Franz diffusion cell analysis was utilized to assess skin permeation and retention, selected for its relevance to topical drug delivery evaluation.
Key Steps: 5-FU-loaded SLN gel and 5-FU plain gel (2 mg 5-FU equivalent) were applied to hairless Wistar rat skin mounted on Franz cells (1.76 cm² area, 12 mL receptor volume) with PBS (pH 5.5) at 37 ± 2°C and 300 rpm. Samples (1 mL) were withdrawn at 0.5, 1, 2, 4, 8, 12, and 24 hours, replaced with fresh PBS, and analyzed at 266 nm. Post-permeation, skin was rinsed, frozen, cut, extracted with 20 mL methanol overnight, sonicated for 30 minutes, and centrifuged at 12,000 rpm for 20 minutes to quantify retained 5-FU.
Data Collection and Analysis: Permeation (% drug permeated, flux, enhancement ratio) and retention (% drug retained, target efficiency) were calculated, with triplicate data analyzed via t-test (p < 0.05).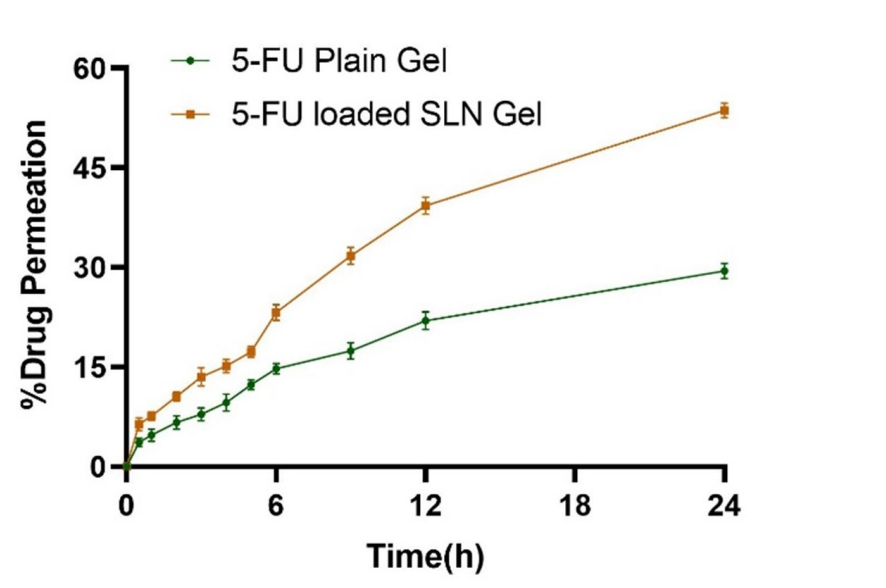
Figure 2. Ex-vivo permeation analysis of 5-FU-loaded SLNs gel and 5-FU plain gel.
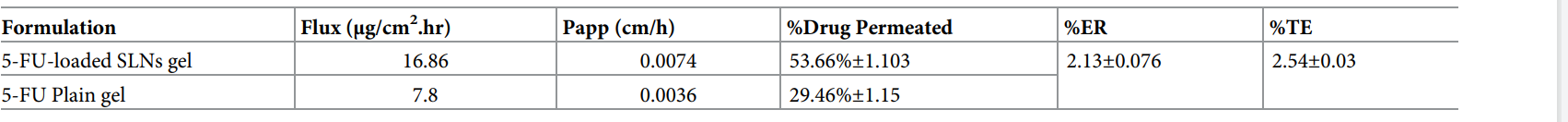
Table 3. Permeation analysis of 5-FU-loaded SLNs gel and 5-FU plain gel
Result: SLN gel showed 53.66 ± 1.103% permeation (flux: 16.86 µg/cm²/h, ER: 2.13 ± 0.076) and 36.75 ± 0.298% retention (TE: 2.54 ± 0.03) versus 29.46 ± 1.15% permeation (flux: 7.8 µg/cm²/h) and 14.47 ± 0.43% retention for plain gel (p < 0.05).
Novel Aspects and Advantages: The SLN gel’s occlusive effect and small size enhance permeation and retention, surpassing traditional gels with limited skin penetration.
Rationale: This experiment was designed to validate topical efficacy, using rat skin to simulate human skin and pH 5.5 to match skin conditions, ensuring clinical relevance.
Conclusion
The successful development of the solid lipid nanoparticle (SLN) drug delivery system for 5-fluorouracil (5-FU) was achieved through a comprehensive approach that included the optimization of formulation parameters, rigorous characterization, and extensive in vitro studies. By utilizing the hot melt encapsulation method, the researchers effectively incorporated 5-FU into lipid matrices, resulting in nanoparticles that exhibited favorable physicochemical properties, including optimal particle size, high entrapment efficiency, and enhanced stability.
The highlights of the study include the demonstration of a biphasic drug release profile, with a significant initial burst followed by sustained release over 48 hours, which is critical for maximizing therapeutic effects while minimizing side effects. Additionally, the SLN formulation exhibited significantly enhanced cytotoxicity against both melanoma and squamous cell carcinoma cell lines compared to free 5-FU, as well as improved cellular uptake and skin permeation. The promising results from the acute toxicity study further indicate the safety and biocompatibility of the developed SLNs, paving the way for potential clinical applications in topical chemotherapy for skin cancer. Overall, this innovative drug delivery system represents a significant advancement in the treatment of skin melanoma and squamous cell carcinoma, addressing the limitations of conventional therapies.
Reference
Ali, Ahsan, et al. “Solid lipid-based nanoparticulate system for sustained release and enhanced in-vitro cytotoxic effect of 5-fluorouracil on skin Melanoma and squamous cell carcinoma.” PLoS ONE, vol. 18, no. 2, 2023, e0281004. doi:10.1371/journal.pone.0281004.
