Editor: Nina
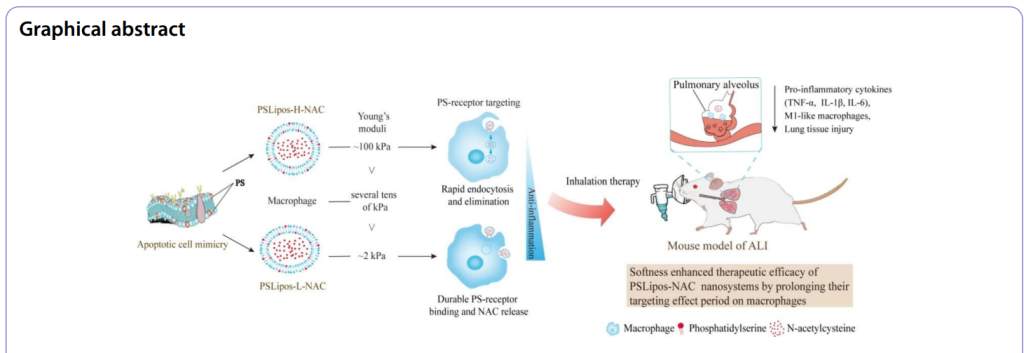
Scientists develop soft, macrophage-targeting apoptotic-cell-inspired nanosystems loaded with N-acetylcysteine to enhance therapeutic efficacy and promote lung repair in acute lung injury.
Key Preview
- Research Question: This study investigates how the elasticity of engineered nanosystems, specifically macrophage-targeting apoptotic-cell-inspired liposomes, affects their therapeutic efficacy in treating acute lung injury (ALI).
- Research Design and Strategy: The research employs a comparative analysis of two types of nanosystems with varying elasticity and their interactions with macrophages in vitro and in vivo.
- Method: The study constructed two types of N-acetylcysteine (NAC)-loaded liposomes with different Young’s moduli, evaluated their macrophage interactions, inflammatory response suppression, and therapeutic effects in a bleomycin-induced ALI mouse model.
- Key Results: The softer liposomes (PSLipos-L-NAC) exhibited a 70-90% suppression of key inflammatory cytokines (TNF-α, IL-1β, IL-6) and significantly improved lung repair in vivo, outperforming the stiffer variants.
- Significance of the Research: This research highlights the importance of nanoparticle elasticity in enhancing macrophage-mediated therapies, offering new insights for designing targeted nanotherapeutics for inflammatory diseases.
Introduction
Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), represent critical clinical syndromes characterized by rapid-onset lung inflammation, alveolar damage, and impaired gas exchange, often triggered by factors such as infection, trauma, or chemical exposure like bleomycin. With high morbidity and mortality rates—exceeding 30-40% in severe cases—ALI poses a significant burden on global healthcare systems, as seen in conditions like COVID-19, where ALI/ARDS is a common lethal manifestation. The inflammatory cascade in ALI is driven by macrophages, which adopt a pro-inflammatory M1 phenotype, releasing mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen species (ROS), exacerbating lung tissue damage.
Traditionally, ALI treatment relies on systemic administration of anti-inflammatory and antioxidant drugs, such as N-acetylcysteine (NAC), delivered via intravenous or oral routes. NAC, a well-established agent, scavenges ROS and reduces cytokine expression, aiming to mitigate inflammation and oxidative stress in the lungs. This approach leverages the drug’s broad bioavailability to address systemic inflammation, often supplemented by supportive care like mechanical ventilation.
However, systemic delivery presents distinct challenges. Poor lung-specific targeting results in low drug concentrations at the injury site, necessitating high doses that increase the risk of systemic side effects, such as nausea or hepatotoxicity. Rapid clearance from circulation further limits efficacy, while the inability to modulate macrophage behavior directly at the alveolar level allows unchecked inflammation to persist. These shortcomings often lead to inadequate therapeutic outcomes, prolonged recovery, and higher mortality rates, underscoring the need for more precise and effective delivery strategies.
To address these limitations, this study introduces an innovative drug delivery approach: inhalable, macrophage-targeting, apoptotic-cell-inspired phosphatidylserine (PS)-containing nano-liposomes (PSLipos) loaded with NAC. By mimicking apoptotic cells, PSLipos exploit PS-receptor interactions to engage lung macrophages, while their tunable elasticity—soft (~2 kPa) versus rigid (~100 kPa)—enhances targeting precision and prolongs therapeutic action. Administered via pulmonary inhalation, this strategy aims to concentrate NAC at the lung injury site, suppress M1 macrophage-driven inflammation, and promote tissue repair, offering a promising advancement over traditional systemic treatments.
Research Team and Aim
The research was led by Dazheng Sun, a scientist affiliated with the Department of Materials Science and Engineering, College of Chemistry and Materials at Jinan University, Guangzhou, China. Conducted in early 2023, this study brought together a multidisciplinary team including Guanglin Zhang, Mingyang Xie, Yina Wang, Xiangchao Liang, Mei Tu, Zhijian Su, and Rong Zeng, with contributors from Jinan University and Shaoguan University. Their collaborative work resulted in the paper titled “Softness enhanced macrophage-mediated therapy of inhaled apoptotic-cell-inspired nanosystems for acute lung injury,” published in the Journal of Nanobiotechnology on May 30, 2023.
The aim of the research, as articulated by Sun in the original paper, was to “construct two kinds of N-acetylcysteine-loaded macrophage-targeting apoptotic-cell-inspired phosphatidylserine-containing nano-liposomes (PSLipos) with different Young’s modulus and investigate their modulus-dependent therapeutic effect on acute lung injury via macrophage-mediated strategy.” This objective reflects the team’s focus on leveraging the biomechanical properties of nanosystems to enhance macrophage-targeted therapy, addressing the critical need for effective ALI treatments through a novel nanotechnology approach.
Experimental Process
Experiment 1: Preparation and Characterization of PSLipos and PSLipos-NAC
Primary Technique: The primary technique used was the combination of thin-film hydration, ultrasonication, and extrusion, followed by a pH gradient method for drug loading, to create phosphatidylserine (PS)-containing nano-liposomes (PSLipos) with varying elasticity and encapsulate N-acetylcysteine (NAC).
Key Steps: Two types of PSLipos were prepared: high-modulus (PSLipos-H, ~100 kPa) and low-modulus (PSLipos-L, ~2 kPa). For PSLipos-H, soybean phosphatidylcholine (SPC), 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine (DPPS), and cholesterol (Chol) were dissolved in a 6.5:1.5:2 molar ratio in chloroform/ethanol (5:1, v/v), evaporated at 37°C to form a lipid film, and hydrated with PBS (pH 7.4). For PSLipos-L, sodium deoxycholate (SDC) was added (Chol:SDC = 1:0.4 molar ratio) to the hydration solution. Both suspensions were sonicated (150 W, 3 min) and extruded through a 100 nm polycarbonate membrane. NAC was loaded into pre-formed liposomes using a pH gradient method: blank liposomes were prepared in SDC/NaHCO₃-NaOH buffer (pH 10.4), dialyzed to pH 7.4, and incubated with NAC (10 mg/mL) at 52°C for 2 h. Liposomes were purified by ultrafiltration (3000 g, 10 min).
Data Collection and Analysis: Particle size, polydispersity index (PDI), and zeta potential were measured using a Zetasizer. Encapsulation efficiency (EE%) and drug loading efficiency (DLE%) were calculated via UV-Vis spectrophotometry at 207 nm, using equations: EE% = [(total NAC – unentrapped NAC) / total NAC] × 100% and DLE% = [(total NAC – unentrapped NAC) / total liposome mass] × 100%. Morphology was assessed by transmission electron microscopy (TEM) after staining with 2% sodium phosphotungstate. Young’s modulus was determined using atomic force microscopy (AFM) based on the Hertz model.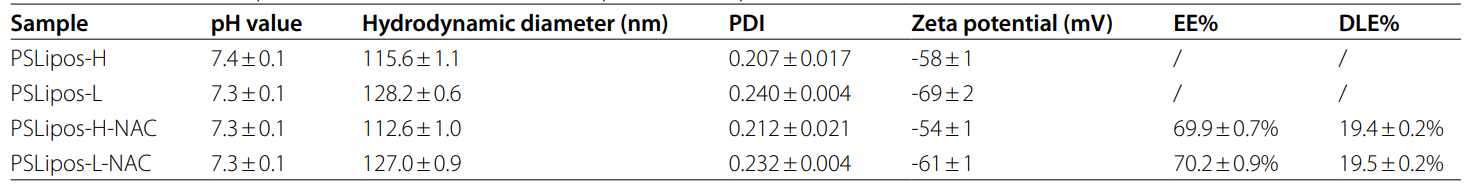
Table 1. The size, zeta potential, EE% and DLE% of PSLipos and PSLipos-NAC with different modulus.
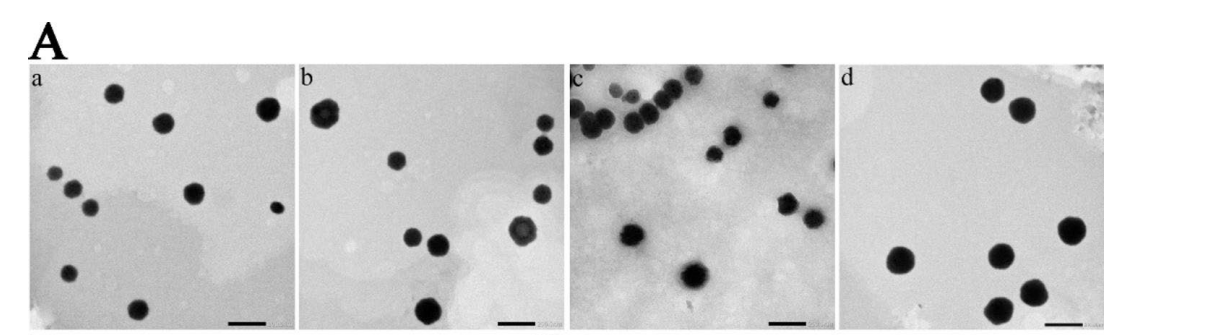
Figure 1. (A) Transmission electron microscopy images. a, b, c and d represent PSLipos-H, PSLipos-L, PSLiposH-NAC and PSLipos-L-NAC, respectively (scale bar = 200 nm).
Result: PSLipos-H and PSLipos-L had similar sizes (~120 nm), PDI (0.25), and zeta potentials (-60 mV), with EE% of 69.9 ± 0.7% and 70.2 ± 0.9%, and DLE% of 19.4 ± 0.2% and 19.5 ± 0.2%, respectively. TEM confirmed spherical morphology (~100 nm), and AFM showed modulus values of ~100 kPa (PSLipos-H) and ~2 kPa (PSLipos-L).
Novel Aspects: The use of SDC as an edge activator to tune elasticity (lowering modulus to ~2 kPa) is innovative, enhancing flexibility compared to traditional rigid liposomes. This modulus control, combined with the pH gradient method, ensures high NAC loading under mild conditions, avoiding harsh processing that could degrade the drug, unlike conventional thin-film hydration alone.
Experiment 2: In Vitro Drug Release and Stability Testing
Primary Technique: The primary technique was dialysis in Gamble’s solution to assess NAC release, coupled with accelerated and long-term stability tests to evaluate liposome robustness.
Key Steps: PSLipos-NAC (H and L) dispersions were placed in dialysis tubing (MWCO 10 kDa) and submerged in Gamble’s solution (pH 7.4) at 37°C with stirring (100 rpm). At set intervals (e.g., 0, 2, 4, 8, 12, 24, 48 h), 2 mL of release medium was sampled and replenished. For stability, liposomes underwent centrifugation (3200 g, 1 h, 25°C), mechanical stirring (180 beats/min, 48 h, 37°C), or storage at 4°C for 30 days.
Data Collection and Analysis: NAC concentration in release samples was measured by UV-Vis spectrophotometry at 207 nm. Stability was assessed by monitoring pH, particle size, PDI, and zeta potential using a Zetasizer, with macroscopic appearance observed visually.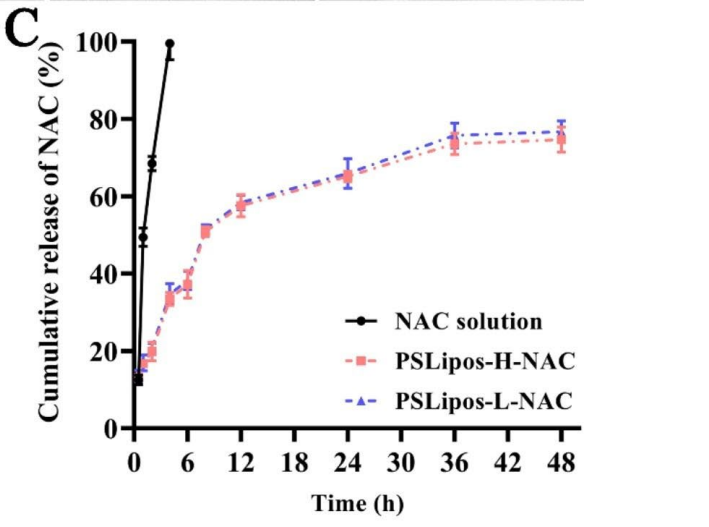
Figure 2. The in vitro NAC release profiles of free NAC and PSLipos-NAC with different modulus.
Result: Both PSLipos-H-NAC and PSLipos-L-NAC exhibited slow NAC release, with ~30% retained after 48 h, showing no significant modulus-dependent difference. Stability tests revealed minimal changes in pH (~7.3-7.4), size (~120 nm), PDI (0.25), and zeta potential (-60 mV) under all conditions.
Novel Aspects: Using Gamble’s solution, mimicking lung interstitial fluid, provides a physiologically relevant release profile, unlike standard PBS used in traditional studies. The stability of soft liposomes (PSLipos-L) under mechanical stress highlights their robustness, a leap over fragile deformable liposomes.
Experiment 3: Macrophage Capture and Intracellular Distribution
Primary Technique: The primary technique was fluorescence-based tracking using flow cytometry and confocal microscopy to study modulus-dependent macrophage interactions with PSLipos.
Key Steps: RAW264.7 cells (10⁴/well) were incubated with fluorescein-DHPE-labeled PSLipos (0.5 µmol/mL total lipids, 6:1000 dye:lipid ratio) for 24 h. For capture, cells were washed and analyzed by flow cytometry. For distribution, cells were treated for 3 h, washed, and cultured up to 24 h, with fluorescence quantified by a microplate reader and imaged at 3 and 12 h via confocal microscopy after staining with DIL (10 µM).
Data Collection and Analysis: Mean fluorescence intensity (MFI) was calculated from flow cytometry (≥3 replicates). Fluorescence over time was measured by microplate reader, and confocal images were taken with a Zeiss LSM 880 to visualize PSLipos localization.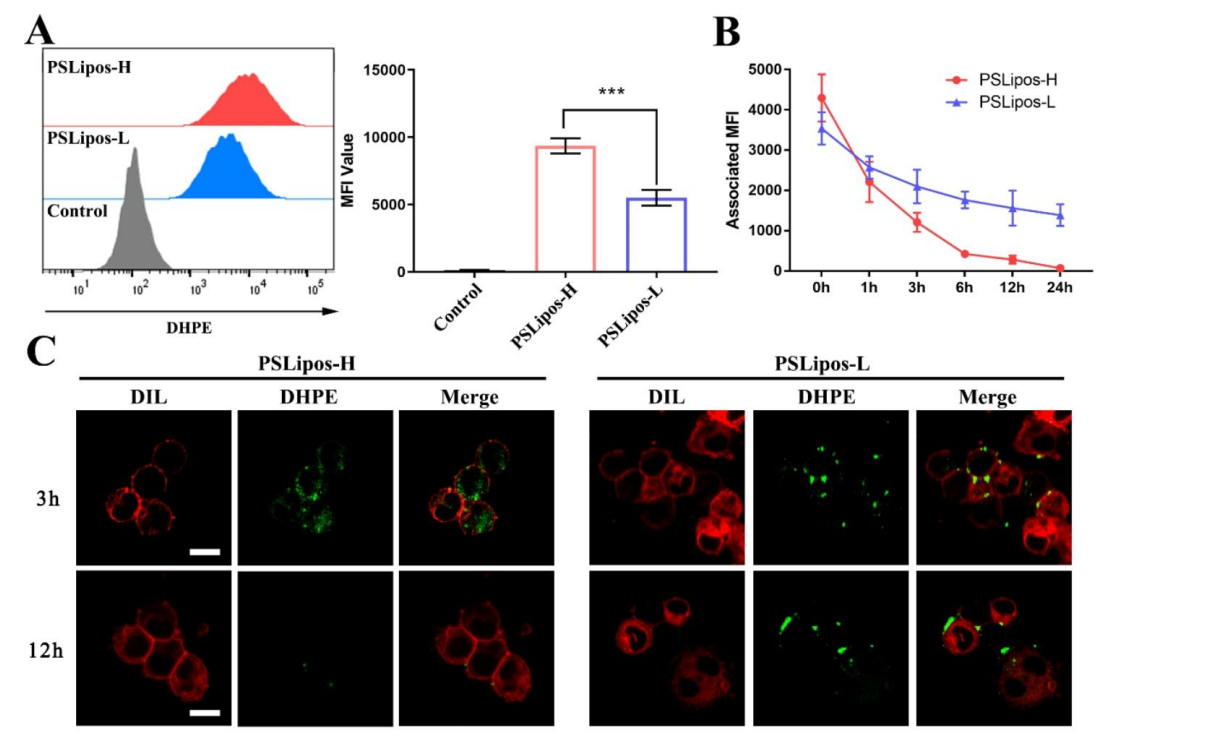
Figure 3. The fate of PSLipos-NAC nanosystems incubated with Raw264.7 cells. (A) Flow cytometry analysis of Raw264.7 cells after 24 h incubation with PSLipos-H and PSLipos-L. The MFI value represents the amount of PSLipos captured by the cells. (B-C) Distribution of PSLipos in Raw264.7 cells. PSLipos were co-cultured with cells for 3 h before being replaced with fresh medium to maintain the culture for a period of time. The MFI values were detected at the indicated times (B). The distribution of PSLipos after 3 and 12 h was analyzed by confocal microscopy ©. Cell membranes were marked in red by DIL. The scale bar represents 10 μm.
Result: PSLipos-L showed ~40% less capture than PSLipos-H (MFI reduction, p < 0.05). PSLipos-H fluorescence dropped to near zero by 24 h, while PSLipos-L retained ~50% signal. Confocal imaging confirmed PSLipos-L remained on the macrophage surface at 12 h, unlike internalized and degraded PSLipos-H.
Novel Aspects: This experiment uniquely demonstrates that softness (low modulus) prolongs surface binding, resisting phagocytosis, unlike traditional rigid nanoparticles that are rapidly internalized, offering a new paradigm for sustained macrophage targeting.
Experiment 4: In Vitro Anti-Inflammatory and Pro-Healing Assays
Primary Technique: The primary technique was a transwell co-culture system of BMDMs and MLE-12 cells to evaluate macrophage-mediated effects of PSLipos-NAC on inflammation and wound healing.
Key Steps: BMDMs (2 × 10⁴/well, upper chamber) and MLE-12 cells (5 × 10⁵/well, lower chamber) were cultured for 24 h, stimulated with LPS (500 ng/mL, 12 h), and treated with NAC (122.5 µg/mL), PSLipos (1 mM lipids), or PSLipos-NAC (1 mM lipids, 122.5 µg/mL NAC) for 24 h. Supernatants were analyzed for cytokines (TNF-α, IL-1β, IL-6) by ELISA and NO by Griess assay. For wound healing, MLE-12 monolayers were scratched, treated as above, and imaged at 0 and 24 h.
Data Collection and Analysis: Cytokine levels were quantified using ELISA kits, NO via absorbance at 540 nm against a NaNO₂ standard curve, and wound closure by ImageJ analysis of scratch area (migration rate = [(A₀ – Aₜ) / A₀] × 100%). Data were analyzed with ANOVA (p < 0.05).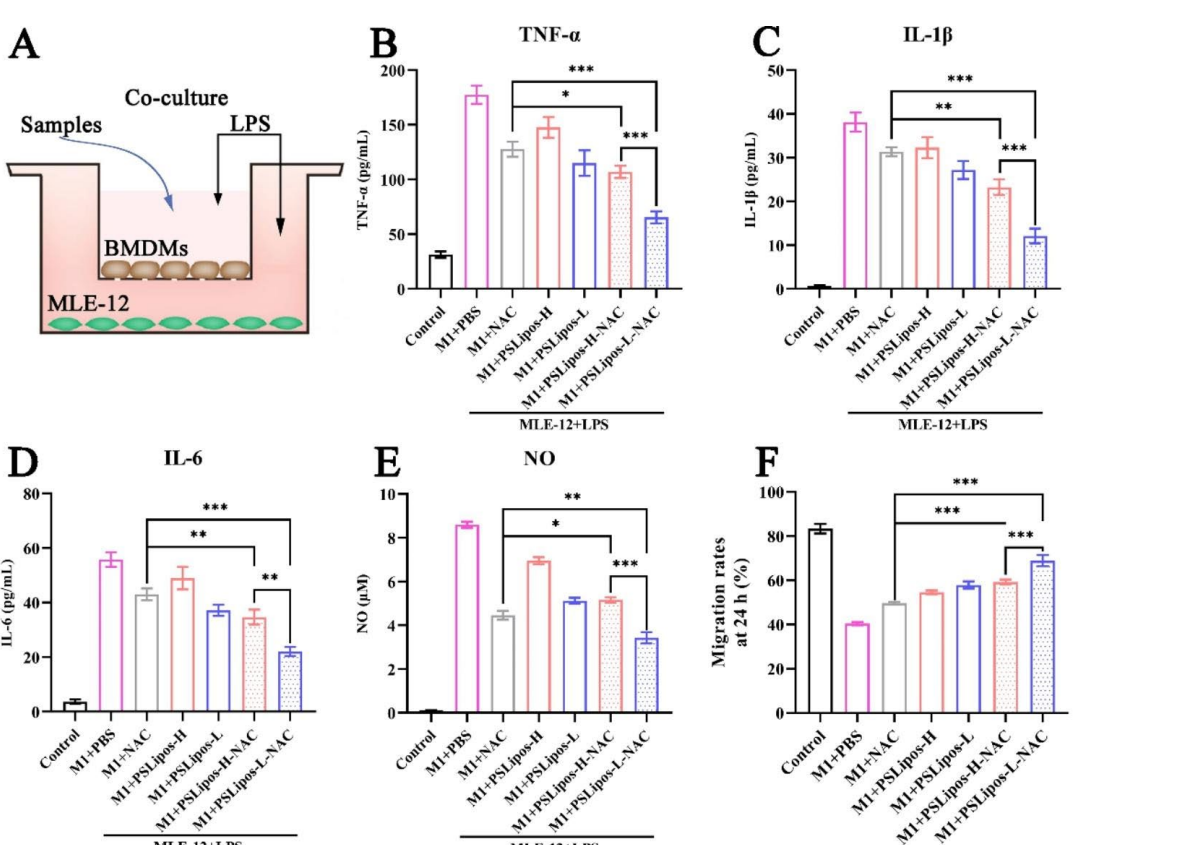
Figure 4. PSLipos-L-NAC more effectively improved wound healing in lung epithelial cells via macrophage-mediated way. (A) Schematics of inflammatory BMDMs and MLE-12 co-cultured system. The pro-inflammatory cytokines expression of (B) TNF-α, © IL-1β, (D) IL-6 in co-culture system was detected after treating with samples using ELISA (n>3). (E) NO production
Result: PSLipos-L-NAC reduced TNF-α, IL-1β, and IL-6 by ~63%, 68%, and 60%, respectively, and NO by ~60%, outperforming PSLipos-H-NAC (~40% reduction, p < 0.01). Migration rate increased to ~70% with PSLipos-L-NAC vs. ~50% in LPS controls (p < 0.001).
Novel Aspects: The co-culture model uniquely assesses macrophage-epithelial crosstalk, revealing softness-enhanced anti-inflammatory and pro-healing effects, surpassing traditional NAC delivery by leveraging sustained surface interactions.
.
Experiment 5: In Vivo Assay for Treating ALI
Primary Technique: The primary technique was pulmonary administration of PSLipos-NAC via aerosol inhalation in a bleomycin-induced ALI mouse model to assess therapeutic efficacy.
Key Steps: Male BALB/C mice (n = 8/group) were intratracheally instilled with bleomycin (2.5 U/kg in 50 µL saline). After 2 h, PBS, NAC (1.8 mg/mL), PSLipos (15 mM lipids), or PSLipos-NAC (15 mM lipids, 1.8 mg/mL NAC) were nebulized (4 mL/mouse, ~3.9 µm droplets) using a Yuwell 403D nebulizer. Mice were sacrificed after 24 h, and lungs/serum were collected.
Data Collection and Analysis: Lung tissue RNA was analyzed by qPCR for TNF-α, IL-1β, IL-6, and iNOS expression (ΔΔCt method, GAPDH normalized). Serum cytokines were measured by ELISA. H&E-stained lung sections were scored (0-1 scale) for injury, and iNOS expression was quantified by immunohistochemistry (ImageJ).
Result: PSLipos-L-NAC reduced lung inflammatory gene expression to 20-40% of BLM levels (p < 0.001) and serum cytokines significantly (p < 0.01). Lung injury scores dropped to the lowest values (~0.2 vs. ~0.8 in BLM, p < 0.001), and iNOS expression was most suppressed (~30% of BLM, p < 0.01).
Novel Aspects: Inhalation of soft PSLipos-L-NAC offers superior ALI attenuation over traditional NAC inhalation, leveraging modulus-dependent macrophage modulation, a novel approach for pulmonary nanotherapy.
Conclusion
The successful development of the inhalable N-acetylcysteine (NAC)-loaded, apoptotic-cell-inspired phosphatidylserine (PS)-containing nano-liposome (PSLipos) drug delivery system was achieved through a meticulous combination of advanced nanotechnology and biomechanical engineering. The research team employed a hybrid method integrating thin-film hydration, ultrasonication, and extrusion to fabricate PSLipos with precisely controlled elasticity—high-modulus PSLipos-H (~100 kPa) and low-modulus PSLipos-L (~2 kPa)—followed by a pH gradient technique to efficiently encapsulate NAC under mild conditions. This approach ensured high drug loading (encapsulation efficiency ~70%) and stability, while the incorporation of sodium deoxycholate (SDC) as an edge activator enabled the tuning of liposome softness, mimicking the flexibility of apoptotic cells. The pulmonary administration via aerosol inhalation further optimized delivery, concentrating NAC at the lung injury site to target macrophages effectively. Rigorous in vitro and in vivo testing, including co-culture models and a bleomycin-induced acute lung injury (ALI) mouse model, validated the system’s ability to modulate macrophage responses and promote lung repair, demonstrating its therapeutic potential.
A key highlight of the study is the finding that “softness may enhance ligand-directed macrophage-mediated therapeutic efficacy of nanosystems,” as evidenced by PSLipos-L-NAC’s superior performance. With a Young’s modulus of ~2 kPa, softer than macrophages, PSLipos-L resisted phagocytosis, prolonged surface binding to PS-receptors, and synergistically combined sustained NAC release with anti-inflammatory signaling. This resulted in a ~60-68% reduction in pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and a ~70% wound healing rate in vitro, alongside significant attenuation of lung injury in vivo (injury score ~0.2 vs. ~0.8 in controls, p < 0.001). These outcomes underscore the critical role of elasticity in enhancing the efficacy of macrophage-targeted nanotherapeutics, offering a groundbreaking advancement over traditional rigid nanoparticle systems and systemic NAC delivery for ALI treatment.
Reference
Sun, Dazheng, et al. “Softness Enhanced Macrophage-Mediated Therapy of Inhaled Apoptotic-Cell-Inspired Nanosystems for Acute Lung Injury.” Journal of Nanobiotechnology, vol. 21, no. 172, 30 May 2023, doi:10.1186/s12951-023-01930-2.
