Editor: Tiffany
A recent study identifies lnc-MLETA1 as a pivotal exosomal long noncoding RNA that promotes lung cancer metastasis by enhancing cell migration and invasion through specific microRNA interactions.
Key Highlights
- Research Question:
What role does exosomal long noncoding RNA (lncRNA) play in the metastasis of lung cancer? - Research Difficulties:
The function and mechanisms of exosomal lncRNA in lung cancer metastasis were largely unexplored, creating a need for innovative experimental approaches. - Key Findings:
The study found that lnc-MLETA1 promotes lung cancer cell migration and invasion by regulating the miR-186-5p/EGFR and miR-497-5p/IGF1R signaling pathways. - Innovative Aspects:
The research identifies lnc-MLETA1 as a significant exosomal lncRNA that facilitates communication between lung cancer cells to enhance metastasis. - Importance of the Study:
This study highlights lnc-MLETA1 as a potential prognostic biomarker and therapeutic target for lung cancer, which is critical given the disease’s high mortality rate due to metastasis.
Impact of lnc-MLETA1 on Lung Cancer Metastasis
Lung cancer, particularly non-small cell lung cancer (NSCLC), poses a significant global health challenge, being the most common and deadliest type of cancer worldwide. Approximately 85% of lung cancer cases fall under the NSCLC category, which is often asymptomatic in its early stages. Consequently, many patients are diagnosed only after the disease has advanced, leading to a dismal five-year survival rate of less than 10%. The primary cause of mortality associated with lung cancer is metastasis, which accounts for approximately 90% of lung cancer-related deaths. This alarming statistic underscores the necessity for in-depth research into the mechanisms that drive metastasis, enabling the development of more effective diagnostic tools and targeted therapeutic strategies. Understanding the molecular interplay between cancer cells and their microenvironment—particularly the role of exosomes and noncoding RNAs—may yield critical insights into lung cancer progression and metastasis.
Objectives of the Study on lnc-MLETA1
The primary aim of this study is to investigate the role of exosomal long noncoding RNA (lncRNA) MLETA1 in promoting metastasis in lung cancer. Specifically, the study seeks to elucidate how lnc-MLETA1 influences cell migration and invasion by acting through specific signaling pathways and miRNA interactions. The objectives of this research include: (1) identifying the expression levels of lnc-MLETA1 in various lung cancer cell lines and exosomes derived from these cells; (2) assessing the functional impact of lnc-MLETA1 on the migratory and invasive capabilities of lung cancer cells through in vitro assays; (3) elucidating the molecular mechanisms by which lnc-MLETA1 regulates key signaling pathways, particularly those involving EGFR and IGF1R, through its interaction with miR-186-5p and miR-497-5p; and (4) evaluating the potential of lnc-MLETA1 as a prognostic biomarker and therapeutic target by correlating its expression with clinical outcomes in lung cancer patients.
Experimental Design and Key Findings
(1) Experimental Process Outline
- Purification of cell exosomes from conditioned media using differential ultracentrifugation.
- Characterization of exosomes through transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA).
- Identification of differentially expressed lncRNAs via lncRNA sequencing (lncRNA-seq) of isolated exosomes.
- Assessment of the effects of exosomes on lung cancer cell migration and invasion using wound-healing assays and transwell invasion assays.
- Overexpression and knockdown of lnc-MLETA1 in lung cancer cell lines to analyze its functional role in cell motility.
- Analysis of lnc-MLETA1’s interaction with specific miRNAs through RNA pulldown assays and MS2-tagged RNA affinity purification.
- Evaluation of the expression levels of EGFR and IGF1R in relation to lnc-MLETA1 using Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR).
- In vivo experiments using orthotopic xenograft models to assess the impact of lnc-MLETA1 on tumor metastasis.
(2) Key Experiments
1. Exosome Characterization
Procedure: Exosomes were purified from the conditioned media of CL1-0 and CL1-5 lung cancer cell lines using differential ultracentrifugation. The purified exosomes were characterized by transmission electron microscopy (TEM) to visualize their morphology, and nanoparticle tracking analysis (NTA) was employed to measure their size distribution and concentration.
Result: TEM revealed cup-shaped vesicles characteristic of exosomes, while NTA provided size distribution metrics showing that exosomes derived from CL1-0 and CL1-5 cells were approximately 133.5 ± 64.2 nm and 140.0 ± 74.5 nm in diameter, respectively.
Finding: The successful characterization of exosomes confirmed that they were appropriately isolated from the lung cancer cell lines, establishing a foundation for further functional assays.
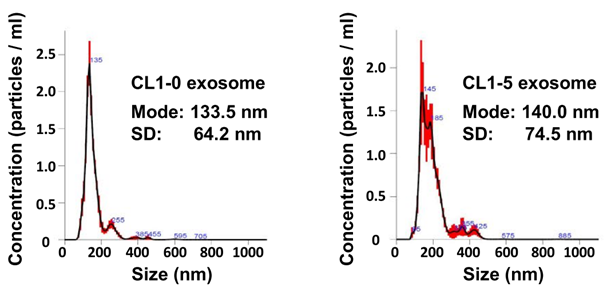
Figure 1. Nanoparticle tracking analysis of the size distributions and number of exosomes derived from CL1-0 and CL1-5 cells.
2. Functional Role of lnc-MLETA1
Procedure: CL1-0 cells were transfected with lnc-MLETA1 overexpression plasmids, while CL1-5 cells were subjected to lnc-MLETA1 knockdown via lentivirus-based short hairpin RNAs (shRNAs). The effects of these manipulations on cell migration and invasion were evaluated using wound-healing assays and transwell invasion assays.
Result: Overexpression of lnc-MLETA1 in CL1-0 cells markedly increased their migratory and invasive capabilities, as evidenced by enhanced wound closure and a higher number of invaded cells in transwell assays. Conversely, knockdown of lnc-MLETA1 in CL1-5 cells resulted in significantly reduced migration and invasion compared to control cells.
Finding: These results indicate that lnc-MLETA1 plays a critical role in promoting lung cancer cell motility and supports the hypothesis that it is a significant factor in lung cancer metastasis.
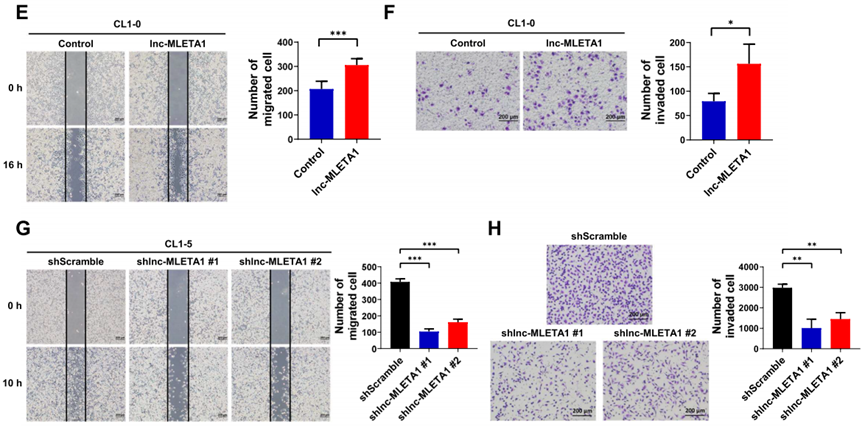
Figure 2. Wound-healing and transwell invasion assays of lnc-MLETA1-overexpressing (CL1-0) and knockdown (CL1-5) cells vs. controls. Scale bar, 200 μm.
3. lnc-MLETA1’s Interaction with miRNAs
Procedure: RNA pulldown assays and MS2-tagged RNA affinity purification were employed to identify and confirm the interaction between lnc-MLETA1 and specific microRNAs (miR-186-5p and miR-497-5p). The expression levels of these miRNAs were analyzed pre- and post-transfection to assess changes in their abundance.
Result: The assays revealed that lnc-MLETA1 bound significantly to miR-186-5p and miR-497-5p, as evidenced by the increased levels of these miRNAs in the pulldown samples. Overexpression of lnc-MLETA1 led to a decrease in the levels of these miRNAs in lung cancer cells.
Finding: This interaction suggests that lnc-MLETA1 functions as a competing endogenous RNA (ceRNA), sponging miR-186-5p and miR-497-5p, thereby modulating the expression of downstream targets, specifically EGFR and IGF1R, to promote cell motility and metastasis.
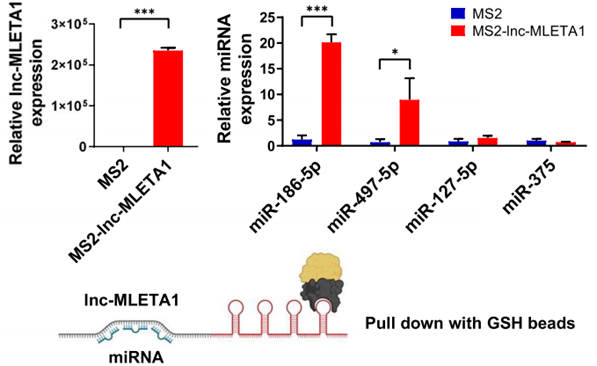
Figure 3. qRT-PCR analysis of lnc-MLETA1 and selected miRNAs (miR-186-5p, miR-497-5p, miR-127-5p, miR-375) in the MS2-TRAP pull-down sample.
Role of lnc-MLETA1 in Cancer Progression and Prognosis
This study establishes lnc-MLETA1 as a critical exosomal long noncoding RNA that significantly contributes to lung cancer metastasis by enhancing cell migration and invasion. Upregulated in highly metastatic lung cancer cell lines and their secreted exosomes, lnc-MLETA1 functions as a competing endogenous RNA (ceRNA), sponging microRNAs miR-186-5p and miR-497-5p, which leads to the upregulation of key signaling pathways mediated by epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (IGF1R). The findings highlight the innovative role of lnc-MLETA1 in facilitating intercellular communication and suggest its potential as a prognostic biomarker, as elevated levels correlate with poor survival outcomes in lung cancer patients. Overall, this research underscores the importance of lnc-MLETA1 in lung cancer progression and opens avenues for targeted therapeutic strategies.
Reference:
Hsu, Xiu-Rui, et al. “Exosomal long noncoding RNA MLETA1 promotes tumor progression and metastasis by regulating the miR-186-5p/EGFR and miR-497-5p/IGF1R axes in non-small cell lung cancer.” Journal of Experimental & Clinical Cancer Research 42.1 (2023): 283.
