Editor: Nina
Scientists develop dual-functional mesoporous silica nanoparticles loaded with Isorhamnetin and anti-PD-L1 antibodies to enhance immune checkpoint inhibition and inhibit YY1-mediated tumor progression in hepatocellular carcinoma.
Key Preview
- Research Question: The study investigates the efficacy of combining Isorhamnetin (ISO) and anti-PD-L1 monoclonal antibody within mesoporous silica nanoparticles to improve the tumor immune microenvironment and inhibit YY1-mediated progression of hepatocellular carcinoma (HCC).
- Research Design and Strategy: The researchers employed a dual-functional nanoparticle approach that combines targeted drug delivery with immune checkpoint inhibition. They focused on the role of transcription factor YY1 and its interaction with deubiquitination enzyme USP7 to understand their contributions to tumor progression and immune evasion.
- Method: The research utilized a variety of experimental techniques, including protein interaction assays, cell culture, in vitro cytotoxicity tests, and animal models to evaluate the effects of the nanoparticles on HCC progression and immune microenvironment.
- Key Results: The study found that the dual-functional nanoparticles significantly inhibited tumor growth in vivo, reduced the presence of myeloid-derived suppressor cells (MDSCs), and enhanced T-cell infiltration within tumors. Quantitatively, treatment with HMSN-ISO@ProA-PD-L1 Ab led to better outcomes than control treatments.
- Significance of the Research: This research provides a new strategy for HCC treatment by targeting YY1-mediated pathways while simultaneously leveraging immune checkpoint inhibition, thereby enhancing therapeutic efficacy and improving the tumor immune microenvironment.
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent form of liver cancer and is a leading cause of cancer-related mortality worldwide. The disease often presents at an advanced stage due to a lack of early symptoms, resulting in a poor prognosis for patients. Conventional treatment options for HCC include surgical resection, liver transplantation, and locoregional therapies, such as transarterial chemoembolization (TACE). Systemic therapies, including chemotherapy and targeted therapies, have also been employed; however, their effectiveness is often limited by the inherent resistance of HCC cells to these treatments.
A common strategy in traditional drug delivery for HCC involves the use of systemic chemotherapy, which aims to target tumor cells throughout the body. However, this approach presents significant challenges. The tumor microenvironment in HCC is often immunosuppressive, which not only reduces the efficacy of chemotherapy but also allows the tumor to evade immune surveillance. Furthermore, the non-specific nature of traditional drug delivery methods can lead to adverse systemic side effects, where healthy tissues are damaged alongside cancer cells, resulting in a diminished quality of life for patients.
To address these challenges, innovative drug delivery strategies have emerged, focusing on targeted and localized therapies that enhance the therapeutic efficacy while minimizing side effects. One such approach involves the use of dual-functional nanoparticles, designed to encapsulate therapeutic agents and immune checkpoint inhibitors. These nanoparticles can deliver drugs directly to tumor sites, improving the bioavailability of the therapeutic agents and promoting a synergistic effect between chemotherapy and immunotherapy. By specifically targeting the tumor microenvironment, this strategy aims to overcome the limitations associated with conventional treatment methods and improve patient outcomes in HCC.
Research Team and Aim
The study was conducted by an interdisciplinary team led by Dr. Huijuan Liu, a prominent researcher at Nankai University. The research was carried out in 2023 and was published in the Journal of Nanobiotechnology. The paper is titled “Isorhamnetin and anti-PD-L1 antibody dual-functional mesoporous silica nanoparticles improve tumor immune microenvironment and inhibit YY1-mediated tumor progression.”
The aim of the research, as articulated by Dr. Liu, was to explore a novel therapeutic strategy that combines the regulation of YY1-mediated tumor progression with immune checkpoint inhibition, specifically through the use of dual-functional mesoporous silica nanoparticles. This innovative approach seeks to enhance the efficacy of hepatocellular carcinoma (HCC) treatment by targeting key molecular pathways associated with tumor growth and immune evasion.
Experimental Process
Primary Technique: Co-immunoprecipitation and Protein Interaction Assays
The main method employed in this research was co-immunoprecipitation (Co-IP) coupled with mass spectrometry, which allowed for the identification of proteins that interact specifically with the transcription factor YY1. This foundational technique is essential for understanding the molecular mechanisms of YY1 regulation through its interaction with deubiquitinating enzyme USP7.
Key Steps: Co-immunoprecipitation
- Preparation of Cell Lysates: PLC-PRF-5 cells expressing Flag-YY1 were cultured and harvested. The cells were lysed using a lysis buffer containing 0.3% Nonidet P-40, 0.2 mM EDTA, 50 mM Tris-HCl (pH 7.4), and 150 mM NaCl, supplemented with a protease inhibitor cocktail.
- Immunoprecipitation: Anti-Flag affinity beads were added to the cell lysates and incubated overnight at 4 °C to allow for the binding of Flag-YY1 and its interacting proteins.
- Washing and Elution: The beads were washed five times with cold lysis buffer to remove unbound proteins. The protein complexes were eluted using Flag peptide and subsequently analyzed by SDS-PAGE followed by mass spectrometry.
Data Collection and Analysis
The presence of YY1 and USP7 in the protein complex was verified through Western blot analysis. The data were collected quantitatively by measuring the intensity of the bands corresponding to YY1 and USP7 after exposure to specific antibodies. Additionally, statistical analysis was performed to assess the significance of the protein interactions, with a p-value of < 0.05 considered significant.
Result: Protein Interaction Confirmation
The results demonstrated a specific interaction between YY1 and USP7, as evidenced by the co-immunoprecipitation of both proteins. Mass spectrometry analysis confirmed the identity of USP7 as a binding partner of YY1, and further experiments revealed that disruption of USP7 led to a significant decrease in YY1 protein levels, confirming the stabilizing role of USP7 on YY1.
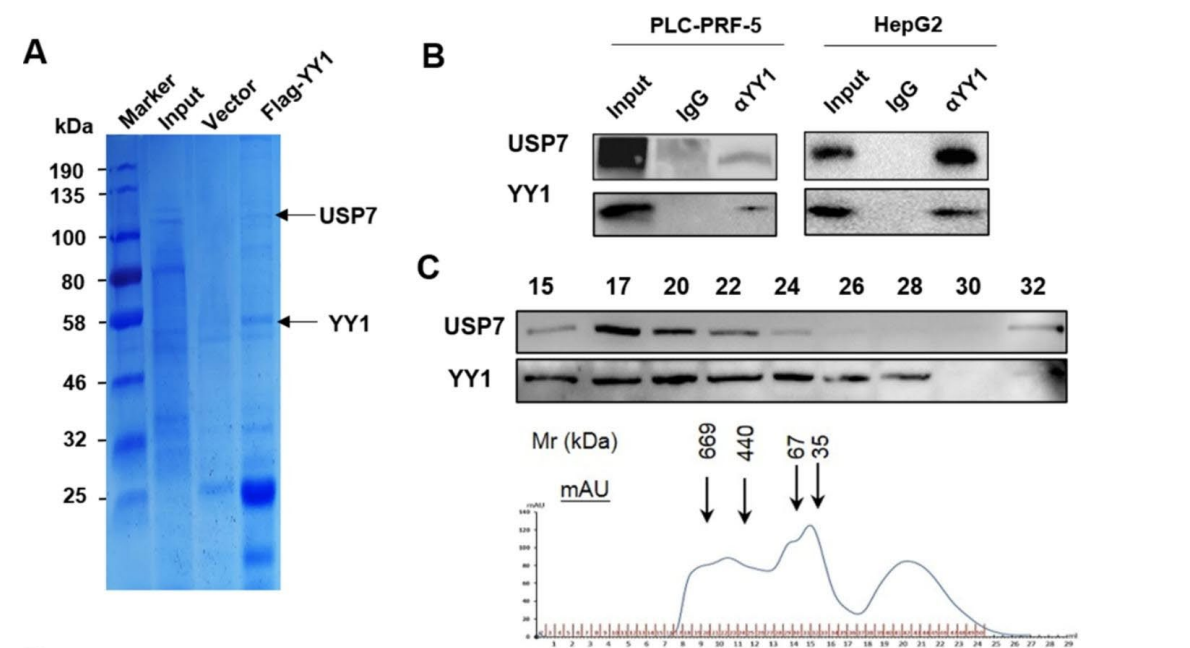
Figure 1. (A) Interaction protein of YY1 in PLC-PRF-5 cells as detected by Flag pull-down affinity-purification experiment. The protein samples were stained with Coomassie Blue, and the proteins interacting with YY1 were detected by MS. (B) USP7 and YY1 interaction detected by Co-IP. © Western blot analysis of the USP7 and YY1 contents in each component of the whole-cell extracts of PLC-PRF-5 separated by FPLC.
Novel Aspects
This study introduced a dual-functional mesoporous silica nanoparticle (HMSN-ISO@ProA-PD-L1 Ab) system that not only serves as a drug delivery vehicle but also enhances the bioavailability of Isorhamnetin (ISO) while providing targeted delivery of anti-PD-L1 antibodies. Unlike traditional nano-delivery systems, which often lack specificity and can lead to systemic side effects, this innovative approach combines targeted drug delivery with immune checkpoint blockade, creating a synergistic therapeutic effect against HCC.
Key Steps: Drug Screening and Activity Assays
- Virtual Screening: Various compounds were screened using computational methods to identify potential USP7 inhibitors, focusing on their binding affinity to the USP7 active site.
- Cell Viability Assays: PLC-PRF-5 cells were treated with identified compounds, including ISO, at varying concentrations to assess cytotoxicity using CCK-8 assays after 24 and 48 hours of incubation.
- Western Blot Analysis: Following treatment with ISO, Western blotting was performed to evaluate the expression levels of YY1 and other EMT markers (E-cadherin and vimentin) to determine the impact of ISO on YY1-mediated EMT.
Data Collection and Analysis
Data were collected from the CCK-8 assays by measuring absorbance at 450 nm. Western blot analysis provided quantitative data on protein expression levels, which were analyzed using ImageJ software. Statistical comparisons were made using one-way ANOVA, with post-hoc tests to assess differences among treatment groups.
Result: Inhibition of YY1 and EMT Markers
ISO treatment resulted in a dose-dependent decrease in YY1 expression and an increase in E-cadherin levels, indicating inhibition of the EMT process. The cell viability assays showed that ISO significantly reduced the proliferation of HCC cells, particularly at higher concentrations.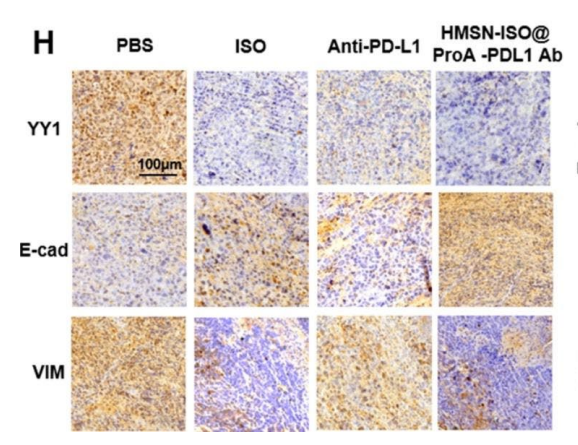
Figure 2. IHC analysis of changes in YY1, vimentin, and E-cadherin levels in tumor tissues after drug treatment.
Novel Aspects
The identification of ISO as a specific inhibitor of USP7 presents a novel therapeutic strategy that not only targets YY1 but also enhances the effectiveness of immune checkpoint inhibitors. This dual functionality of the nanoparticle system marks a significant advancement over traditional therapies by addressing both tumor progression and immune evasion simultaneously.
Key Steps: Nanoparticle Synthesis and Characterization
- Synthesis of HMSN: Amine-functionalized mesoporous silica nanoparticles (A-HMSN) were synthesized, characterized for size and surface area, and subsequently loaded with ISO.
- Antibody Functionalization: The surface of A-HMSN was modified with Protein A to facilitate the binding of anti-PD-L1 antibodies, creating HMSN-ISO@ProA-PD-L1 Ab.
- Characterization of Nanoparticles: The size, zeta potential, and morphology of the nanoparticles were analyzed using Dynamic Light Scattering (DLS) and transmission electron microscopy (TEM).
Data Collection and Analysis
Characterization data were collected for particle size and zeta potential to ensure appropriate loading and functionalization. Statistical analysis was performed for size distribution, with results confirming successful synthesis and modification of nanoparticles.
Result: Successful Nanoparticle Synthesis
The synthesized HMSN-ISO@ProA-PD-L1 Ab exhibited a significant increase in size due to drug and antibody loading, with zeta potential measurements indicating good stability in solution. The release studies demonstrated a controlled release profile of ISO, which was higher in slightly acidic conditions, mimicking the tumor microenvironment.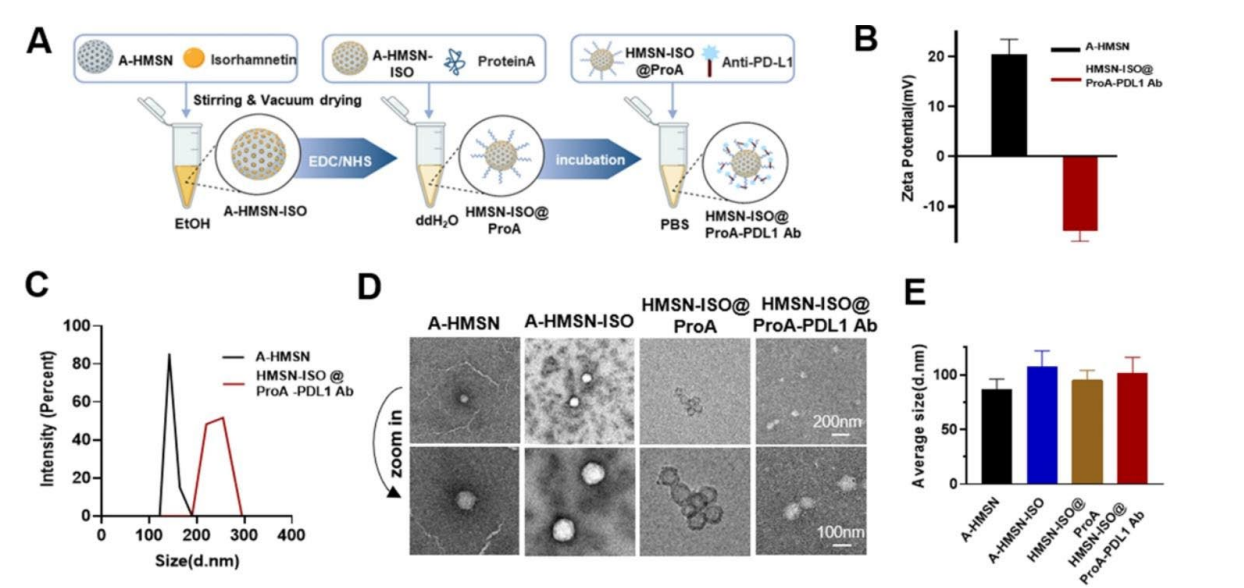
Figure 3. A) Schematic of the preparation of HMSNISO@ProA-PD-L1 Ab. (B, C) Zeta Potential and particle-size distribution of A-HMSN and HMSN-ISO@ProA-PD-L1 Ab as determined by dynamic light scattering. (D) Cryo-TEM image of A-HMSN, A-HMSN-ISO, HMSN-ISO@ProA and HMSN-ISO@ProA-PD-L1 Ab. (E) The statistical results of the average diameter of the particles under TEM.
Novel Aspects
The innovative use of Protein A for antibody loading on mesoporous silica nanoparticles marks a significant advancement in targeted drug delivery systems, providing a more effective means of delivering both therapeutic agents and immune modulators directly to tumor cells while minimizing off-target effects.
Key Steps: In Vivo Efficacy Studies
- Xenograft Model: Hepa1-6 cells were subcutaneously implanted into BALB/c mice to establish xenograft tumors. Once tumors reached approximately 100 mm³, treatment with HMSN-ISO@ProA-PD-L1 Ab was initiated.
- Treatment Administration: Mice were treated intratumorally with HMSN-ISO@ProA-PD-L1 Ab, ISO, or anti-PD-L1 antibody alone, with tumor size measured every three days.
- Analysis of Tumor Microenvironment: Post-treatment, tumors were harvested, and flow cytometry was used to analyze the presence of myeloid-derived suppressor cells (MDSCs) and T-cell infiltration.
Data Collection and Analysis
Tumor volume was measured with calipers, and flow cytometry data were analyzed using appropriate gating strategies to quantify immune cell populations. Statistical significance between treatment groups was determined through ANOVA.
Result: Enhanced Antitumor Efficacy
The results indicated that treatment with HMSN-ISO@ProA-PD-L1 Ab significantly inhibited tumor growth compared to control groups, with flow cytometry revealing a marked reduction in MDSCs and an increase in CD8+ T-cell populations in the tumor microenvironment.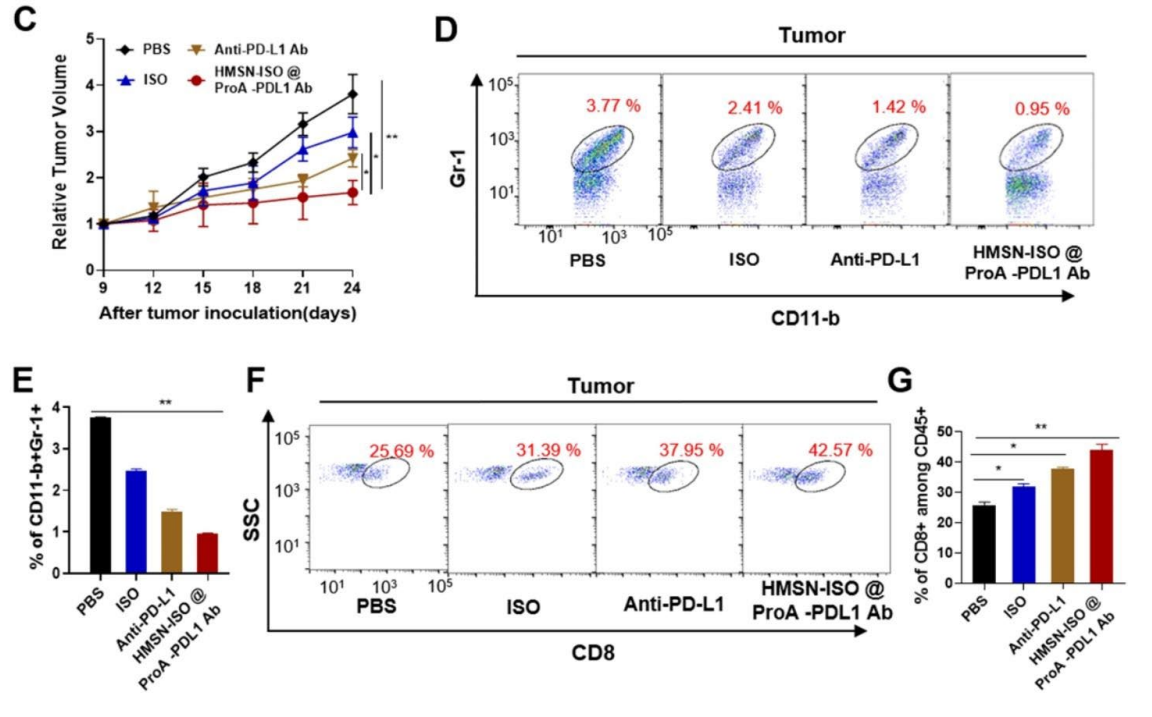
Figure 4. (D, E) Flow cytometry analysis of the proportion of myeloid-derived suppressor cells (MDSCs) in tumor tissues after drug treatment. (F, G) Flow cytometry analysis of the proportion of CD8 + T-cells in tumor tissues.
Novel Aspects
This study’s use of a dual-functional nanoparticle platform to deliver both a chemotherapeutic agent and an immune checkpoint inhibitor represents a groundbreaking approach to cancer treatment. The ability to target tumors specifically while modulating the immune response showcases a significant improvement over conventional therapies that often lack specificity and efficacy.
Through these meticulously designed experiments, this research provides a replicable framework for exploring the dual targeting of tumor progression and immune evasion in HCC, offering a promising direction for future therapeutic strategies.
Conclusion
The successful development of the dual-functional drug delivery system, HMSN-ISO@ProA-PD-L1 Ab, was achieved through a series of meticulously designed experiments that combined innovative nanoparticle synthesis with targeted drug delivery and immune modulation strategies. By encapsulating Isorhamnetin (ISO) and anti-PD-L1 antibodies within mesoporous silica nanoparticles, the research team was able to create a system that not only effectively delivers therapeutic agents to tumor sites but also enhances immune checkpoint inhibition. This dual approach addresses the challenges posed by traditional drug delivery methods in the treatment of hepatocellular carcinoma (HCC).
The highlights of the study include the identification of the interaction between the transcription factor YY1 and the deubiquitinating enzyme USP7, which plays a critical role in stabilizing YY1 and promoting tumor progression. Furthermore, the research demonstrated that ISO could target USP7, promoting YY1 ubiquitin-dependent degradation and thereby inhibiting epithelial-mesenchymal transition (EMT). The in vivo studies provided compelling evidence that the HMSN-ISO@ProA-PD-L1 Ab nanoparticles significantly inhibited tumor growth, reduced myeloid-derived suppressor cells (MDSCs), and enhanced T-cell infiltration in the tumor microenvironment.
Overall, this research opens new avenues for more effective HCC therapies by integrating targeted drug delivery with immune modulation, thereby improving therapeutic outcomes and patient quality of life.
Reference
Liu, Huijuan, et al. “Isorhamnetin and anti-PD-L1 antibody dual-functional mesoporous silica nanoparticles improve tumor immune microenvironment and inhibit YY1-mediated tumor progression.” Journal of Nanobiotechnology 21.1 (2023): 208.
