Editor: Tiffany
A novel approach combining high-intensity focused ultrasound with biomimetic oxygen-carrying nanoparticles significantly enhances tumor destruction and immune response in cancer treatment, addressing key challenges in traditional therapies.
Key Highlights
- Research Question:
Can a novel oxygen-carrying nanoparticle combined with high-intensity focused ultrasound and immunotherapy improve cancer treatment outcomes? - Research Difficulties:
HIFU’s effectiveness is often limited by energy attenuation within tissues and the presence of residual tumors that can lead to recurrence. - Key Findings:
The synergistic effect of nanoparticles (M@P-SOP) with HIFU significantly enhances tumor destruction and stimulates a robust immune response, leading to reduced tumor growth and metastasis. - Innovative Aspects:
The use of biomimetic nanoparticles that target tumor cells while also providing oxygen to improve the tumor microenvironment is a novel approach in cancer therapy. - Importance of the Study:
This research presents a promising strategy for enhancing HIFU treatments and improving immunotherapy effectiveness, making strides towards more effective cancer therapies.
Challenges in High-Intensity Focused Ultrasound for Cancer Treatment
High-intensity focused ultrasound (HIFU) is increasingly recognized as a non-invasive method for treating solid tumors by delivering concentrated ultrasonic energy that induces thermal ablation and mechanical destruction of tumor tissues. This technique is particularly promising for cancers located in sensitive areas, such as the prostate, breast, and liver, where traditional surgical interventions may pose significant risks. Despite its potential, HIFU faces challenges, primarily due to energy attenuation as ultrasound waves penetrate deeper tissues, limiting effective tumor targeting. Additionally, HIFU treatment often leaves residual tumor cells, raising concerns about recurrence and metastasis. Recent studies have suggested that integrating HIFU with other therapeutic modalities, such as immunotherapy, could enhance its efficacy by leveraging the immune response generated during tumor ablation. However, the presence of a hypoxic tumor microenvironment can inhibit this immune response, necessitating innovative strategies to improve therapeutic outcomes.
Objectives of Combining HIFU with M@P-SOP Nanoparticles
This study aims to explore a novel therapeutic approach that combines high-intensity focused ultrasound (HIFU) with a biomimetic oxygen-carrying nanoparticle (M@P-SOP) to enhance the treatment of solid tumors. The primary objective is to evaluate the effectiveness of this combination in improving tumor destruction and stimulating an immune response while simultaneously mitigating the hypoxic tumor microenvironment. Specifically, the research will examine the following objectives:
- Characterize the biodistribution and targeting efficiency of M@P-SOP nanoparticles in tumor tissues.
- Assess the impact of M@P-SOP on the efficacy of HIFU treatment in preclinical tumor models.
- Investigate the immunological mechanisms activated by the combination therapy, particularly focusing on dendritic cell maturation and CD8+ T cell activation.
- Evaluate the potential for this combined strategy to inhibit tumor growth and prevent metastasis, thereby establishing a novel protocol for cancer therapy that could be translated to clinical settings.
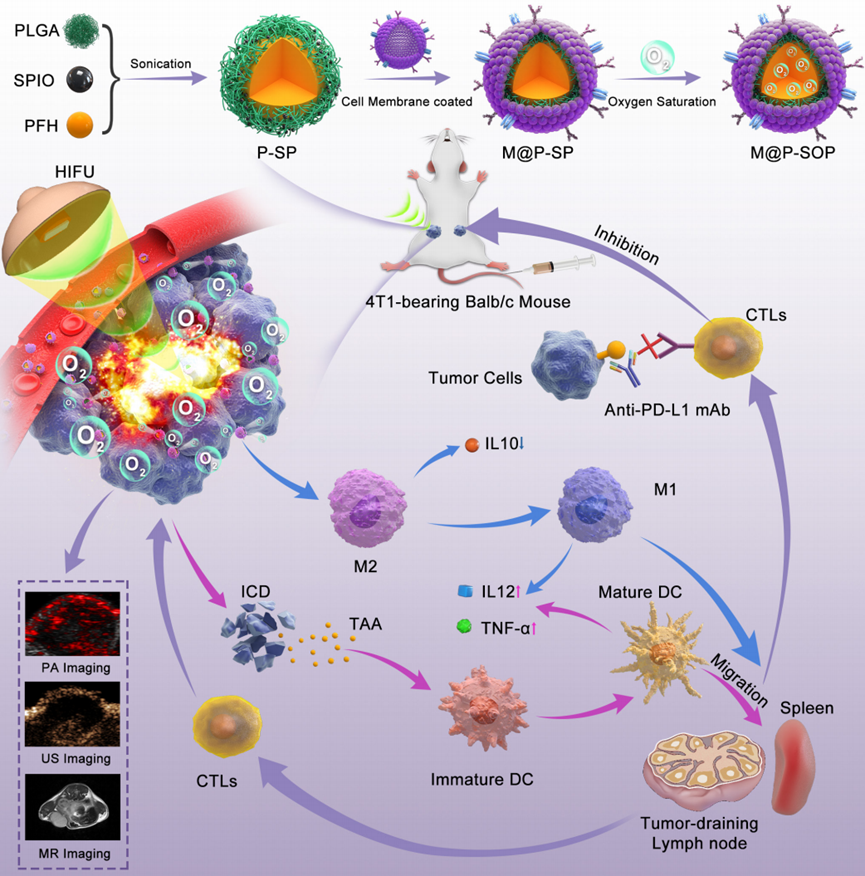
Figure 1. Schematic illustration of the preparation of M@P-SOP, the synergistic effects and mechanism of M@P-SOP-augmented HIFU in combination with anti-PD-L1 blockade against primary and distant tumors.
Experimental Design and Key Findings
(1) Experimental Process Outline
- Preparation of M@P-SOP nanoparticles using the double emulsion and film extrusion method.
- Characterization of M@P-SOP nanoparticles for size, zeta potential, and encapsulation efficiency.
- In vitro studies on the targeting efficiency of M@P-SOP in 4T1 tumor cells.
- In vivo biodistribution studies of DiR-labeled M@P-SOP in tumor-bearing mice.
- Evaluation of multimodal imaging capabilities (ultrasound, MRI, and photoacoustic imaging) of M@P-SOP.
- Ex vivo HIFU ablation studies using fresh bovine liver to assess ablation efficiency.
- In vivo therapeutic efficacy assessment using the unilateral 4T1 tumor model with various treatment groups.
- Analysis of immune response mechanisms through flow cytometry and immunofluorescence.
(2) Key Experiments
1. In Vitro Targeting Efficiency of M@P-SOP
- Procedure: DiI-labeled P@P and M@P-SOP nanoparticles were co-incubated with 4T1 cells in CLSM dishes for varying time intervals (0.5, 1, 2, and 4 hours). The internalization of nanoparticles was assessed using confocal laser scanning microscopy (CLSM) and flow cytometry (FCM).
- Result: M@P-SOP exhibited significantly higher fluorescence intensity compared to P@P, indicating greater internalization in 4T1 tumor cells. Time-dependent internalization was observed for both nanoparticles, with M@P-SOP showing a stronger signal at all time points.
- Finding: The results confirmed that the CCM coating on M@P-SOP enhances its targeting ability, allowing for more effective uptake by homologous tumor cells.
2. In Vivo Biodistribution of M@P-SOP
- Procedure: DiR-labeled M@P-SOP was intravenously administered to mice bearing unilateral 4T1 tumors. Fluorescence imaging was conducted at multiple time points (1, 3, 6, 24, and 48 hours post-injection) to monitor biodistribution and accumulation in tumor tissues.
- Result: The maximum fluorescence intensity was observed at the tumor site after 24 hours, significantly higher than that of the control group (DiR-labeled P@P).
- Finding: M@P-SOP demonstrated effective tumor targeting and accumulation, supporting its potential as a delivery vehicle for HIFU treatment.
3. Evaluation of HIFU Synergistic Ablation with M@P-SOP
- Procedure: Fresh bovine liver tissues were treated with saline, M@P, M@P-S, or M@P-SOP, followed by HIFU exposure at varying power levels (90W, 120W, and 150W) for 3 seconds. The ablation areas were assessed visually and quantitatively through tissue slicing and imaging.
- Result: The M@P-SOP group exhibited significantly larger ablation volumes compared to control groups, indicating enhanced HIFU efficacy.
- Finding: The presence of M@P-SOP markedly improved the ablation effectiveness of HIFU, showcasing its potential as a synergistic agent in tumor treatment.
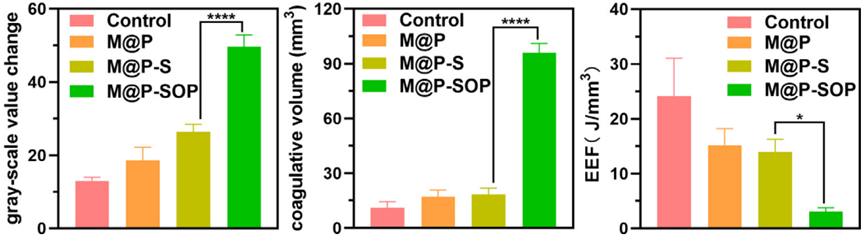
Figure 2. Corresponding gray scale value, necrosis volume and EEF at targeted tumor site after HIFU irradiation.
4. In Vivo Antitumor Efficacy of HIFU Combined with M@P-SOP
- Procedure: Mice with 4T1 tumors were divided into treatment groups: control, M@P-SOP, HIFU, and HIFU combined with M@P-SOP. Tumor growth was monitored, and excised tumors were analyzed for necrosis and immune cell infiltration.
- Result: The HIFU combined with M@P-SOP group showed significant tumor weight reduction compared to other treatment groups, with increased necrotic tissue observed in histological analyses.
- Finding: This experiment demonstrated that the combination of HIFU and M@P-SOP effectively inhibited tumor growth, highlighting a promising therapeutic strategy for cancer treatment.
Implications of Combined HIFU and M@P-SOP Therapy in Cancer Treatment
This study introduces a novel therapeutic strategy that combines high-intensity focused ultrasound (HIFU) with biomimetic oxygen-carrying nanoparticles (M@P-SOP) to improve solid tumor treatment. Key findings reveal that M@P-SOP enhances targeting efficiency and tumor accumulation, significantly boosting HIFU ablation efficacy while inducing a robust immune response characterized by dendritic cell maturation and CD8+ T cell activation. The innovative aspect of this research lies in M@P-SOP’s ability to alleviate hypoxia in the tumor microenvironment, overcoming the immunosuppressive barriers commonly faced in cancer therapies.
The significance of this work is its potential to offer a dual-action treatment approach that integrates physical and immunological mechanisms, maximizing therapeutic benefits with reduced side effects. Additionally, M@P-SOP’s versatility for multimodal imaging allows precise monitoring of tumor responses, enhancing treatment efficacy. Ultimately, the integration of M@P-SOP with HIFU and immune checkpoint blockade presents a promising opportunity for advancing clinical cancer treatment strategies, offering improved management options for a broad range of patients.
Reference:
Tang, Rui, et al. “Novel combination strategy of high intensity focused ultrasound (HIFU) and checkpoint blockade boosted by bioinspired and oxygen-supplied nanoprobe for multimodal imaging-guided cancer therapy.” Journal for ImmunoTherapy of Cancer 11.1 (2023): e006226.
