Editor: Tiffany
A novel therapeutic strategy using M1 macrophage-derived exosomes loaded with the ferroptosis inducer RSL3 has been shown to effectively accumulate in tumors, induce ferroptosis, and reprogram the immunosuppressive tumor microenvironment, leading to enhanced antitumor immunity and significant inhibition of tumor growth in preclinical models.
Key Highlights
- Research Question:
Can engineered macrophage-derived exosomes effectively mitigate the immunosuppressive tumor microenvironment (ITM) and enhance ferroptosis in tumor cells? - Research Difficulties:
Overcoming the limitations of current therapies that fail to retain the functional properties of parent macrophages and efficiently target tumor cells remains a significant challenge. - Key Findings:
The study demonstrated that M1 macrophage-derived exosomes loaded with the ferroptosis inducer RSL3 can significantly enhance oxidative stress in tumor cells, promote immune responses, and effectively reduce tumor progression. - Innovative Aspects:
This research introduces a novel method of utilizing macrophage-derived exosomes, which retain the functional characteristics of their parent cells, thereby improving targeted delivery and efficacy compared to traditional nanovesicle approaches. - Importance of the Study:
The findings pave the way for new therapeutic strategies in cancer treatment that could enhance the effectiveness of immunotherapy, particularly in overcoming challenges posed by the ITM.
Challenges in Cancer Immunotherapy: The Role of the Immunosuppressive Tumor Microenvironment
Cancer treatment continues to face significant obstacles due to the immunosuppressive tumor microenvironment (ITM), a complex network surrounding tumors that hinders effective therapeutic outcomes. The ITM is characterized by immune cells such as M2 macrophages and regulatory T-cells (Tregs), which suppress immune responses, creating a protective barrier that allows tumors to proliferate unchecked. This environment poses a major challenge for current immunotherapies, which are designed to enhance the immune system’s ability to target and eliminate cancer cells. Despite their potential, these therapies often fail to achieve optimal efficacy because the ITM neutralizes immune activation, enabling tumor progression. Consequently, there is a pressing need for innovative approaches to modulate the ITM, shifting it from a tumor-supportive state to one that is hostile to cancer growth. Such strategies could enhance the performance of immunotherapies by overcoming the immunosuppressive barriers within the tumor vicinity. This study introduces a novel nanotechnology-based method aimed at addressing these challenges, focusing on reprogramming the ITM to improve cancer treatment outcomes.
Targeting the Tumor Microenvironment with M1 Macrophage-Derived Exosomes and Ferroptosis Induction
The researchers sought to develop a nanotechnology-based approach to enhance cancer immunotherapy. Their primary aim was to engineer M1 macrophage-derived exosomes loaded with RSL3, a ferroptosis-inducing agent, to simultaneously promote ferroptosis—a type of programmed cell death—and modulate the ITM. The objectives included evaluating the ability of these RSL3-loaded exosomes (RSL3-Exo) to induce ferroptosis in cancer cells, target tumor tissues effectively, and reshape the immunosuppressive environment to favor antitumor immunity. By integrating the tumor-homing properties of exosomes with the therapeutic potential of RSL3, the researchers aimed to create a dual-action strategy for combating cancer. The study, published in Journal for Immunotherapy of Cancer in 2023, provides a detailed investigation into this approach, offering insights into its potential as a next-generation cancer therapy.
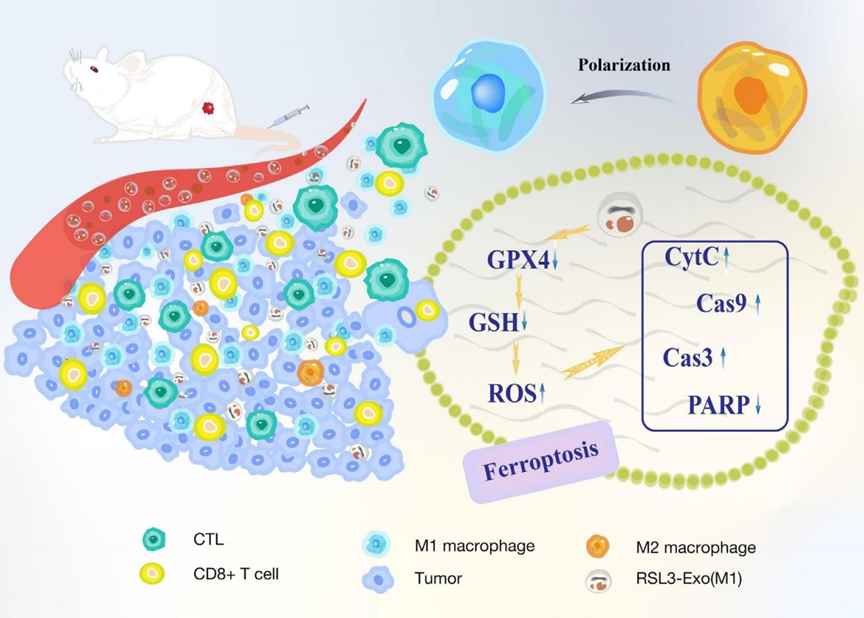
Figure 1. Schematic of macrophage-inherited exosomes mitigating ITM by promoting M1 polarization, downregulating GPX4/GSH, increasing ROS, enhancing CD8+ T/CTL infiltration, and reducing Tregs.
Experimental Validation: From Exosome Engineering to In Vivo Antitumor Efficacy
The researchers conducted a series of experiments to develop and assess the efficacy of RSL3-loaded M1 macrophage-derived exosomes (RSL3-Exo). Below is a summary of the key experimental procedures, followed by detailed descriptions of selected experiments.
General Procedures
- M1-phenotype macrophages were derived from isolated bone marrow cells and used to produce exosomes (M1-Exo), which were subsequently loaded with RSL3 to create RSL3-Exo.
- In vitro assays tested the ferroptosis-inducing capacity of RSL3-Exo on cancer cells, measuring lipid peroxidation, glutathione (GSH) levels, glutathione peroxidase 4 (GPX4) expression, and reactive oxygen species (ROS) accumulation.
- In vivo studies in tumor-bearing mice evaluated the distribution, antitumor efficacy, and immune modulation effects of RSL3-Exo through fluorescence imaging, tumor growth monitoring, and immune cell analysis.
Key Experiments
1. Characterization of Exo and RSL3-Exo
- Procedure: Transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) assessed the size and morphology of Exo and RSL3-Exo, while Western blot analysis confirmed the presence of M1 macrophage proteins.
- Result: Both Exo and RSL3-Exo exhibited a size of approximately 100 nm, with Western blot verifying retention of parental macrophage proteins in the exosomes.
- Finding: Loading RSL3 did not alter exosome size, and the retention of M1 characteristics suggested suitability for targeted delivery.
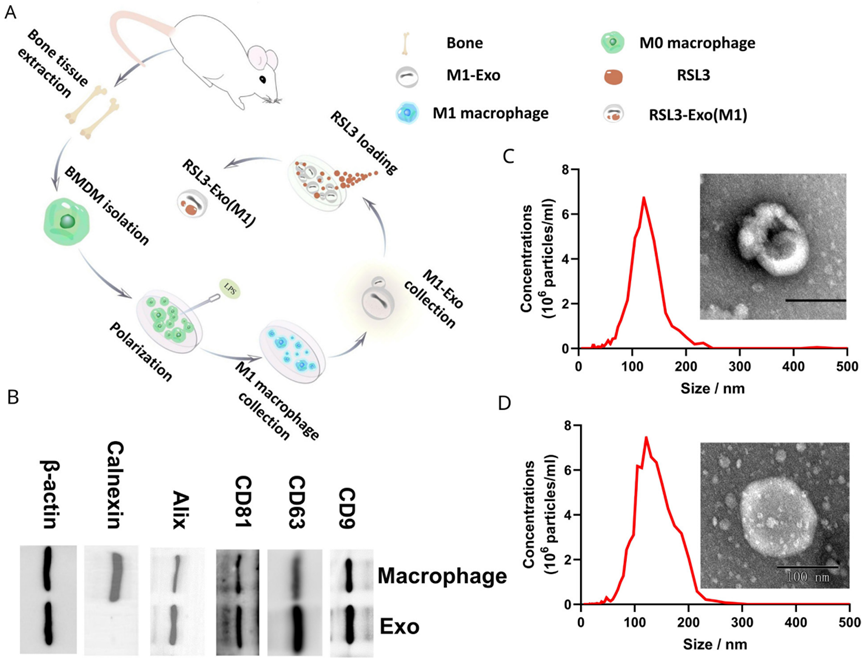
Figure 2. Characterization and test on the Exo and RSL3-Exo.
2. In vitro Ferroptosis Tests
- Procedure: 4T1 tumor cells were treated with Exo, RSL3, or RSL3-Exo, followed by assays for lipid peroxidation (C11 BODIPY 581/591 staining), GPX4 expression (Western blot), and GSH and ROS levels (specific indicators).
- Result: RSL3-Exo significantly increased lipid peroxidation, reduced GPX4 expression and GSH levels, and elevated ROS accumulation compared to controls and RSL3 alone.
- Finding: RSL3-Exo demonstrated enhanced ferroptosis induction in cancer cells, attributed to exosome-mediated delivery.
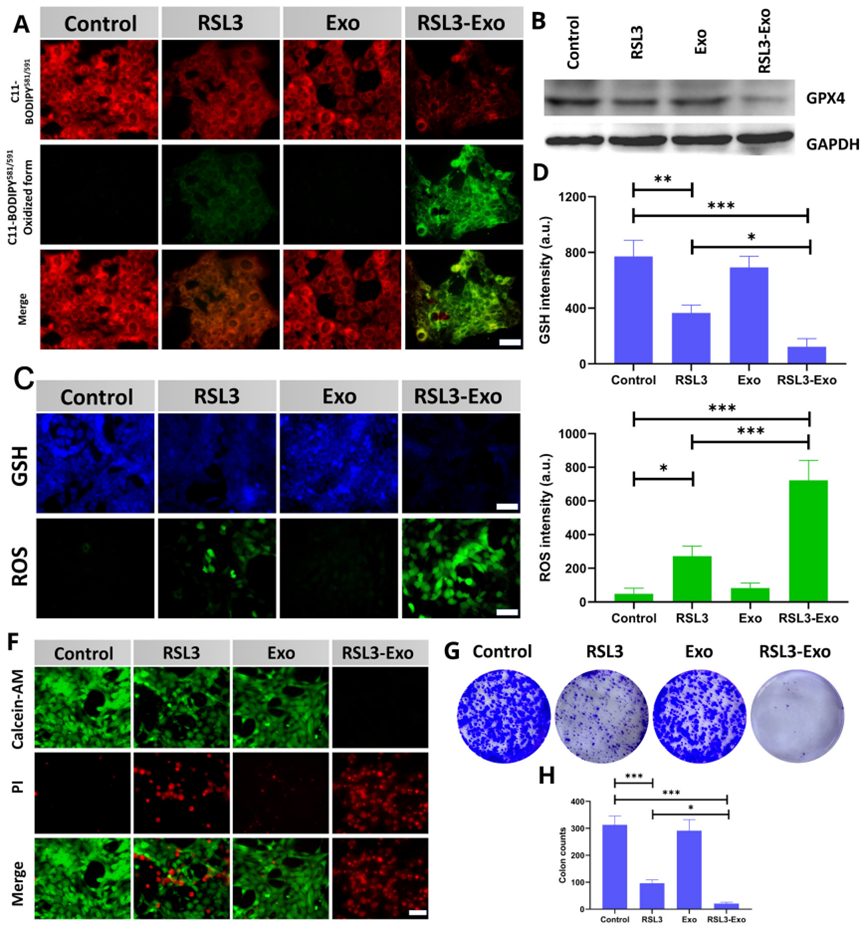
Figure 3. In vitro tests on ferroptosis via detecting its biomarkers or pathological characteristics.
3. In vivo Distribution and Antitumor Efficacy
- Procedure: ICG-labeled Exo were injected into tumor-bearing mice to track distribution via fluorescence imaging, while separate groups received saline, RSL3, Exo, or RSL3-Exo to monitor tumor growth and survival.
- Result: Fluorescence imaging revealed peak exosome accumulation in tumors at 18 hours post-injection. RSL3-Exo treatment resulted in the slowest tumor growth and highest survival rates.
- Finding: The exosome delivery system effectively targeted tumors, enhancing RSL3’s antitumor effects.
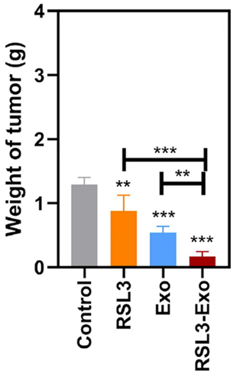
Figure 4. Weights of isolated tumors from 4T1 tumor-bearing mice after the corresponding treatments in each group.
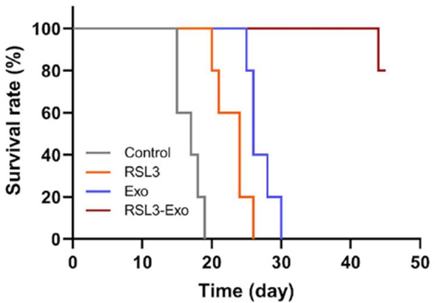
Figure 5. The time-correlated survival rate of 4T1 tumor-bearing mice after the corresponding treatments in each group.
4. Immune Response Evaluations
- Procedure: Tumor tissues were analyzed using flow cytometry and immunofluorescence for tumor-associated macrophage (TAM) polarization, T-cell infiltration, and cytokine levels.
- Result: RSL3-Exo shifted M2 TAMs to M1 TAMs, increased CD8+ T-cell and cytotoxic T-lymphocyte infiltration, reduced Treg presence, and altered cytokine profiles (upregulated proinflammatory, downregulated anti-inflammatory).
- Finding: RSL3-Exo modulated the ITM, promoting an antitumorigenic immune profile alongside ferroptosis induction.
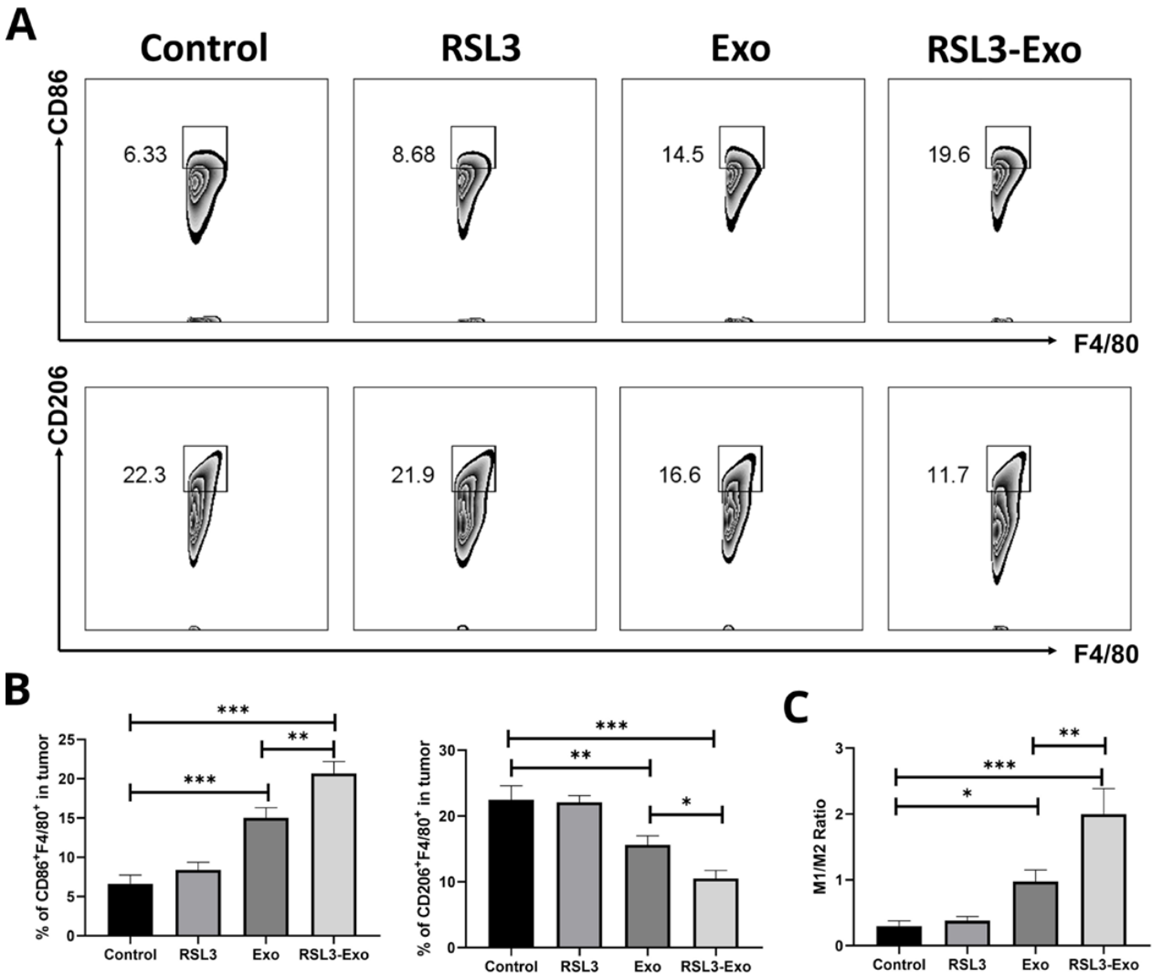
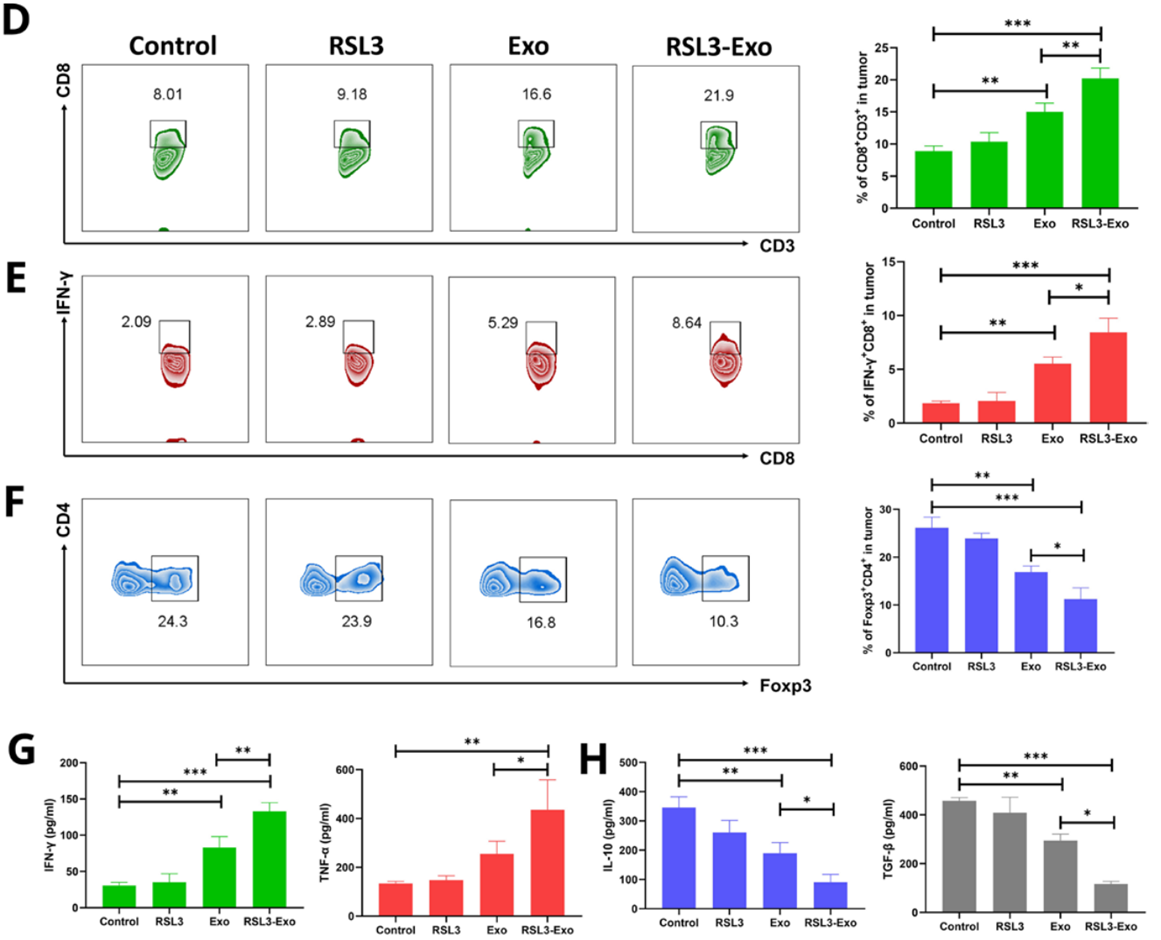
Figure 6. Mechanism exploration on ferroptosis-unlocked ITM alleviation and immune activation by such macrophage inherited exosome executioners.
Implications for Cancer Treatment: Modulating the TME to Enhance Immunotherapy
This study demonstrates that M1 macrophage-derived exosomes loaded with RSL3 (RSL3-Exo) effectively induce ferroptosis in cancer cells while reprogramming the immunosuppressive tumor microenvironment (ITM). The research revealed that RSL3-Exo, leveraging the tumor-homing capabilities of exosomes, achieved significant accumulation in tumor tissues, enhancing ferroptosis through increased lipid peroxidation, reduced GPX4 expression, and elevated ROS levels. Beyond direct cancer cell death, RSL3-Exo modulated the ITM by promoting the conversion of protumorigenic M2 macrophages to antitumorigenic M1 macrophages, reducing regulatory T-cell populations, and increasing CD8+ T-cell and cytotoxic T-lymphocyte infiltration. These changes were accompanied by a shift in cytokine profiles toward a proinflammatory state, further supporting antitumor immunity. The innovation lies in the dual mechanism of RSL3-Exo—inducing ferroptosis and alleviating immunosuppression—addressing a key limitation in cancer immunotherapy. This exosome-based strategy highlights a promising avenue for improving treatment efficacy, suggesting potential applications in developing advanced therapies for various cancers.
Reference:
Wang, Duo, et al. “Macrophage-inherited exosome excise tumor immunosuppression to expedite immune-activated ferroptosis.” Journal for Immunotherapy of Cancer 11.5 (2023): e006516.
