Editor: Tiffany
Researchers have developed a prebiotic nanoparticle that significantly improves the delivery of capecitabine to colorectal cancer tumors while modulating gut microbiota to enhance anti-tumor immunity, offering a promising new approach to CRC therapy.
Highlights
- Research Question:
How can the delivery and efficacy of capecitabine be improved for colorectal cancer treatment while minimizing side effects? - Research Difficulties:
Achieving targeted drug delivery to CRC tumors and prolonging the drug’s half-life without disrupting gut microbiota. - Key Findings:
The SCXN nanoparticle delayed drug clearance, increased intra-tumoral capecitabine concentration, enhanced anti-tumor immunity, and extended survival in CRC mouse models. - Innovative Aspects:
The use of a prebiotic-based nanoparticle to combine chemotherapy with gut microbiota modulation, offering a dual-action approach to CRC treatment. - Importance of the Study:
This study introduces a novel strategy that could improve CRC treatment outcomes by addressing both drug delivery challenges and immune modulation through the gut microbiota.
Challenges in Colorectal Cancer Treatment and the Role of Gut Microbiota
Colorectal cancer (CRC) ranks as the third most diagnosed cancer globally, posing a significant public health challenge. Its prevalence is notably high among individuals under 50 years of age, and it is associated with substantial morbidity and mortality. Symptoms of CRC typically include changes in bowel habits, blood in the stool, abdominal pain, and unexplained weight loss. These symptoms often remain subtle in early stages, complicating timely diagnosis and intervention.
Current treatment options for CRC encompass radiotherapy, chemotherapy, and immunotherapy. Among these, capecitabine (Cap), an oral chemotherapeutic agent, is widely used as a first-line treatment. However, these therapies have notable drawbacks. Radiotherapy and chemotherapy frequently induce adverse side effects due to their nonspecific effects on healthy tissues. Capecitabine, in particular, suffers from rapid clearance from the body and lacks a mechanism for targeted delivery to tumor sites, reducing its efficacy. Furthermore, these treatments can disrupt the gut microbiota, which plays a vital role in regulating immune responses and influencing therapeutic outcomes. Consequently, there is an urgent need for innovative, targeted therapies that enhance efficacy while minimizing toxicity and preserving gut microbiota function.
Developing a Prebiotic Nanoparticle to Enhance Capecitabine Efficacy
This study sought to address the limitations of capecitabine by improving its delivery and efficacy while reducing associated side effects. A central challenge identified was the difficulty in achieving targeted drug delivery to the tumor site and extending the drug’s half-life in vivo. To tackle this, the researchers developed a nanoparticle termed SCXN, composed of a prebiotic xylan-stearic acid conjugate loaded with capecitabine. The primary objectives were to enhance drug accumulation at the tumor, modulate the gut microbiota to bolster anti-tumor immunity, and improve overall treatment outcomes in CRC.
The research was conducted by a team including Tianqun Lang and Runqi Zhu from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, alongside collaborators from other Chinese institutions. Their findings were published in Nature Communications on August 7, 2023.
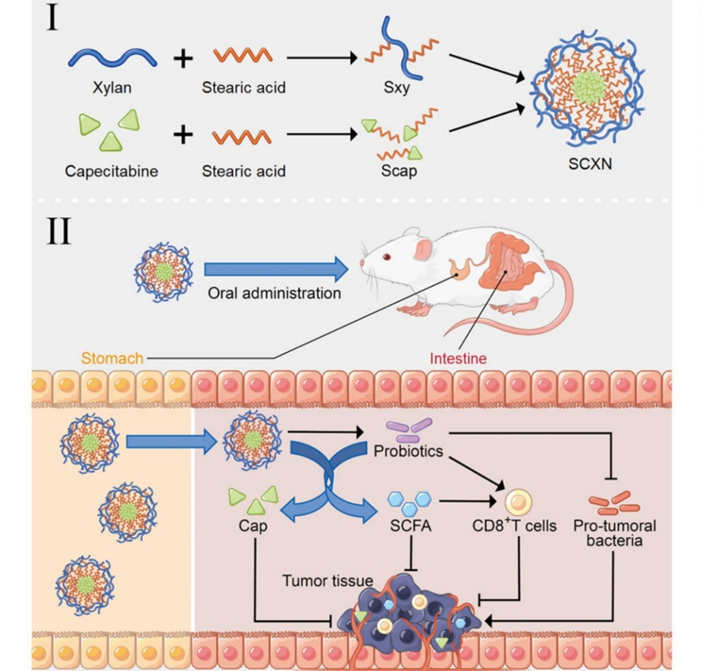
Figure 1. Schematic illustration of combining chemotherapy and gut microbiota regulation for CRC treatment by SCXN.
Experimental Validation of SCXN: From Synthesis to In Vivo Efficacy
The study employed a comprehensive experimental framework to synthesize, characterize, and evaluate the SCXN nanoparticle:
- Synthesis of the xylan-stearic acid conjugate (Sxy) and formulation of SCXN with capecitabine.
- Characterization of SCXN’s physical properties, such as size and drug loading efficiency.
- In vitro experiments to examine drug release kinetics and cytotoxicity against CRC cells.
- In vivo studies in CRC mouse models to assess pharmacokinetics, biodistribution, anti-tumor efficacy, and impacts on gut microbiota and immune responses.
Below are detailed descriptions of key experiments and findings:
1. Synthesis and Characterization of SCXN
- Procedure: The researchers synthesized a xylan-stearic acid conjugate (Sxy) and encapsulated capecitabine to form SCXN. They characterized the nanoparticle using techniques such as dynamic light scattering (DLS) for size and high-performance liquid chromatography (HPLC) for drug loading efficiency.
- Result: SCXN was successfully created with a size and drug encapsulation profile suitable for drug delivery applications.
- Finding: The nanoparticle’s physical properties suggest it is well-suited for delivering capecitabine to CRC tumors, laying the foundation for its therapeutic potential.
2. In Vitro Drug Release and Cytotoxicity
- Procedure: SCXN’s drug release behavior was tested in simulated gastric and intestinal fluids to mimic the gastrointestinal environment. Cytotoxicity was assessed using CT26 CRC cells to evaluate the nanoparticle’s effectiveness against cancer cells.
- Result: SCXN exhibited a sustained release of capecitabine and showed enhanced cytotoxicity compared to free capecitabine.
- Finding: These results indicate that SCXN can release its drug payload in a controlled manner, potentially improving efficacy and reducing toxicity, while being more effective at killing CRC cells than the free drug.
3. In Vivo Pharmacokinetics and Biodistribution
- Procedure: CRC mouse models received oral doses of either free capecitabine or SCXN. Drug concentrations in blood and tissues were measured over time using HPLC to assess pharmacokinetics and biodistribution.
- Result: SCXN delayed the clearance of capecitabine from the blood and increased its concentration in tumor tissues compared to free capecitabine.
- Finding: The nanoparticle enhances the drug’s circulation time and tumor-specific accumulation, improving its pharmacokinetic profile for CRC treatment.
4. Anti-Tumor Efficacy
- Procedure: Mice with CT26-luc tumors were treated with SCXN or control formulations, such as free capecitabine, over three weeks. Tumor growth was monitored using bioluminescence imaging, and survival times were recorded.
- Result: SCXN markedly reduced tumor growth, achieving a tumor inhibition rate of 71.78% compared to 5.29% for free capecitabine, and extended the median survival time from 14 days to 33.5 days.
- Finding: These specific outcomes, directly from the paper, demonstrate SCXN’s superior ability to suppress tumor progression and prolong survival, highlighting its potential as an advanced CRC therapy.
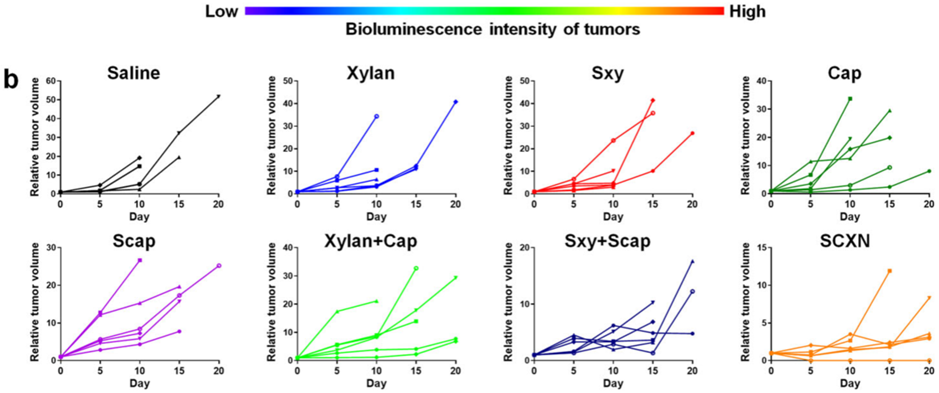
Figure 2. Variation in relative tumor volumes calculated according to the amount of photons during the therapy period.
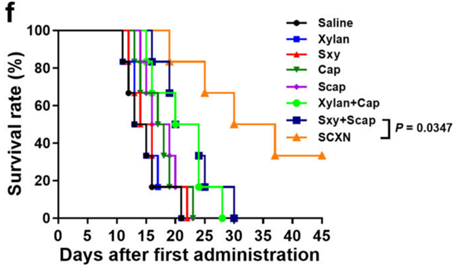
Figure 3. Survival curves of mice within 45 d post the 1st administration.
5. Modulation of Gut Microbiota and Immune Response
- Procedure: Fecal samples from treated mice were analyzed via 16S rDNA sequencing to study gut microbiota changes, while tumor tissues were examined using flow cytometry to assess immune cell infiltration.
- Result: SCXN promoted the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus, and increased CD8+ T cell infiltration in tumors.
- Finding: Beyond delivering capecitabine, SCXN reshapes the gut microbiota to favor probiotic populations and boosts anti-tumor immunity, contributing to its therapeutic efficacy.
A Novel Dual-Action Approach to CRC Therapy
This research demonstrates that the SCXN nanoparticle substantially enhances capecitabine’s therapeutic potential in colorectal cancer treatment. Key findings include prolonged drug clearance, elevated intra-tumoral drug levels, enhanced anti-tumor immunity via gut microbiota modulation, and extended survival in CRC mouse models. The integration of a prebiotic-based nanoparticle with chemotherapy represents a novel strategy, addressing the shortcomings of existing treatments by offering targeted delivery and reduced toxicity. This approach not only improves drug efficacy but also leverages the gut microbiota to strengthen immune-mediated tumor suppression.
Reference:
Lang, Tianqun, et al. “Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy.” Nature Communications 14.1 (2023): 4746.
