Editor: Nina
Scientists develop fluorinated polyamidoamine dendrimer-mediated nanoparticles for targeted delivery of microRNA-23b, effectively reducing inflammation and promoting apoptosis in rheumatoid arthritis models.
Key Preview
Research Question
The study investigates whether fluorinated polyamidoamine dendrimer (FP) can effectively deliver miR-23b to target inflammatory processes in rheumatoid arthritis (RA) models, promoting apoptosis in activated macrophages and reducing inflammation.
Research Design and Strategy
The research employs a therapeutic strategy using FP nanoparticles for miR-23b delivery in experimental RA models (adjuvant-induced arthritis rats), assessing both in vitro and in vivo efficacy.
Method
FP/miR-23b nanoparticles were synthesized and administered intravenously to RA rats. Various assays were conducted to evaluate their anti-inflammatory effects, apoptosis induction, and overall therapeutic efficacy.
Key Results
FP/miR-23b showed significant therapeutic effects including reduced inflammation, improved mobility, and diminished bone damage in RA models, without evident systemic toxicity.
Significance of the Research
This study presents a novel miRNA-based therapeutic approach for RA, demonstrating dual actions of apoptosis induction and inflammatory inhibition, which could lead to better management of autoimmune diseases.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that affects approximately 0.5–1.0% of the global population. Characterized by persistent synovitis, RA leads to joint damage, significant disability, and a decreased quality of life for affected individuals. The disease is marked by the hyperplasia and infiltration of immune cells, particularly macrophages, which release pro-inflammatory cytokines that perpetuate the inflammatory cycle. Despite advancements in treatment modalities, the underlying mechanisms driving RA remain poorly understood, necessitating innovative therapeutic strategies.
Traditionally, the management of RA has focused on the use of disease-modifying antirheumatic drugs (DMARDs) and biologics aimed at suppressing inflammation and modulating the immune response. Common strategies for drug delivery include systemic administration via oral or injectable routes, which target the entire body but often lead to suboptimal drug concentrations at the site of action. While these treatments can alleviate symptoms and slow disease progression for some patients, they frequently fail to address the root causes of inflammation and may not provide long-term efficacy. Furthermore, the systemic nature of these therapies can result in adverse effects and limited patient compliance.
The challenges associated with current RA treatment approaches stem from the complexity of the disease pathology, the heterogeneity of patient responses, and the limitations of drug delivery systems. Many patients do not respond adequately to traditional therapies, and the recurrence of symptoms upon discontinuation of treatment indicates a need for more effective, targeted interventions. Additionally, the risk of systemic toxicity from conventional therapies underscores the necessity for solutions that minimize side effects while maximizing therapeutic outcomes.
To address these challenges, the present study introduces an innovative drug delivery strategy utilizing fluorinated polyamidoamine dendrimer (FP) nanoparticles for the targeted delivery of microRNA-23b (miR-23b). This approach aims to enhance the delivery of therapeutic agents directly to inflamed tissues, promoting localized effects while reducing systemic exposure. By leveraging the unique properties of FP nanoparticles, this strategy seeks to not only suppress inflammation but also induce apoptosis in activated macrophages, offering a dual-action therapeutic solution for improved management of RA and potentially other autoimmune diseases.
Research Team and Aim
The research team comprises Haobo Han, Jiakai Xing, Wenqi Chen, Jiaxin Jia, and Quanshun Li from the School of Life Sciences at Jilin University. The lead researcher, Quanshun Li, conducted this study from February 2021 to February 2023. Their paper, titled “Fluorinated polyamidoamine dendrimer-mediated miR-23b delivery for the treatment of experimental rheumatoid arthritis in rats,” was published in the journal Nature Communications.
The aim of the research, as expressed by lead researcher Quanshun Li, was to “develop an effective miRNA-based therapeutic strategy that not only targets inflammation in RA but also induces apoptosis in the activated macrophages responsible for perpetuating the disease.” This dual-action approach aims to enhance patient outcomes and provide a novel solution for the management of rheumatoid arthritis.
Experimental Process
Primary Technique
The primary technique employed in this study is the synthesis and characterization of fluorinated polyamidoamine (FP) dendrimer nanoparticles for the targeted delivery of miR-23b in rheumatoid arthritis (RA) models. This method integrates nanotechnology with gene therapy, utilizing the unique properties of FP to enhance therapeutic efficacy while minimizing toxicity.
Synthesis and Characterization of FP/miR-23b Nanoparticles
Key Steps
- Synthesis of FP: Hepta-fluorobutyric anhydride was reacted with amine-terminated PAMAM dendrimer in methanol to create FP. The reaction was stirred at room temperature for 48 hours, followed by dialysis against distilled water for 48 hours to remove unreacted materials.
- Characterization: The synthesized FP was characterized using 19F NMR to confirm chemical modification. The degree of fluorination was quantified using a ninhydrin assay to assess the amine groups available on the FP surface.
- Nanoparticle Formation: FP was complexed with miR-23b plasmid at varying N/P ratios to form FP/miR-23b nanoparticles. Gel retardation assays were performed to verify the binding efficiency of the nanoparticles to miR-23b.
Data Collection and Analysis
Characterization data were collected using various spectroscopic techniques, including 19F NMR and MALDI-TOF mass spectrometry for molecular weight determination. The binding affinity of FP to miR-23b was evaluated through gel electrophoresis, which provided quantitative data on complex formation at different N/P ratios.
Results
The synthesis produced FP nanoparticles with a successful degree of fluorination, confirmed by NMR and mass spectrometry. The gel retardation assays demonstrated that FP effectively condensed miR-23b at N/P ratios greater than 2.0, indicating the formation of stable nanoparticles suitable for further investigation.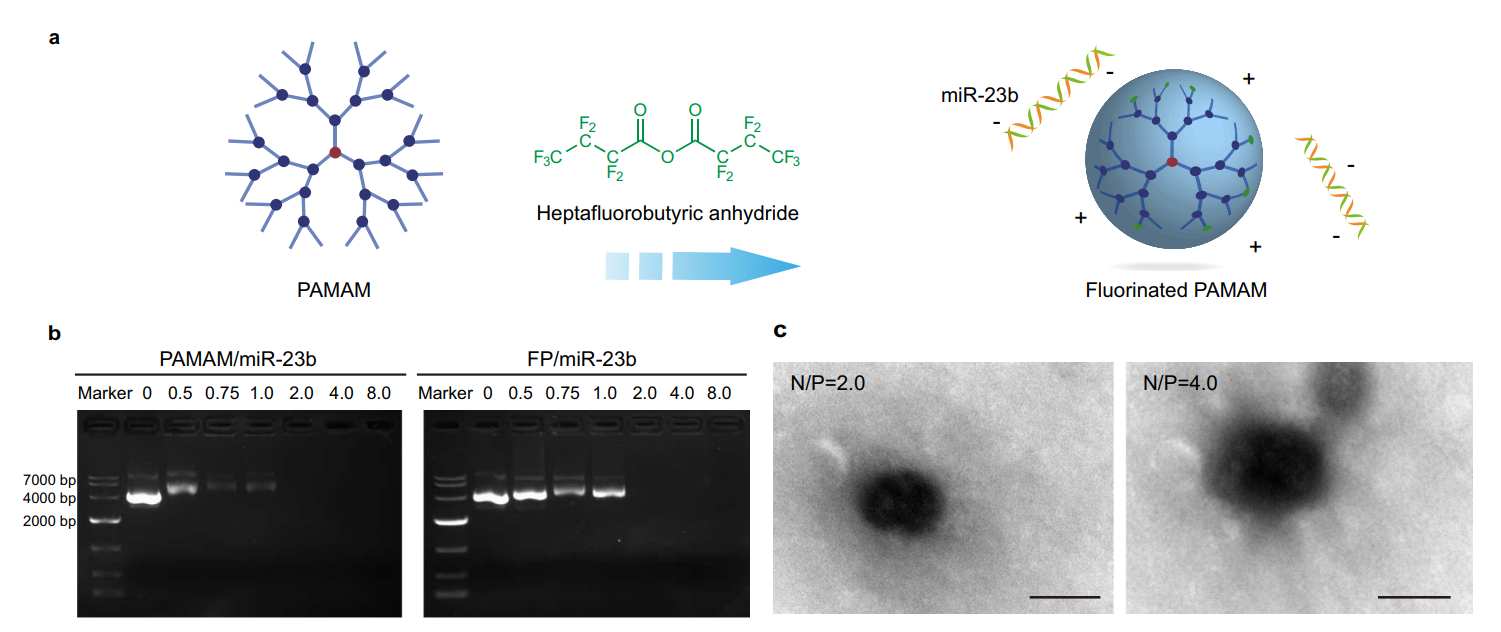
Figure 1. Synthesis and characterization of fluorinated PAMAM (FP)
Novel Aspects
This study introduces a novel fluorination strategy that enhances the transfection efficiency of PAMAM dendrimers, overcoming limitations associated with traditional gene delivery systems. The ability of FP to maintain effective binding while reducing cytotoxicity represents a significant advancement in nanoparticle design for RNA delivery.
In Vitro Evaluation of FP/miR-23b Nanoparticles
Key Steps
- Cell Culture: Murine macrophage cell lines (RAW264.7 and bone marrow-derived macrophages) were cultured and stimulated with LPS to mimic an inflammatory environment.
- Transfection: The FP/miR-23b nanoparticles were administered to the stimulated macrophages at optimized N/P ratios, followed by a 6-hour incubation period.
- Assessment of Transfection Efficiency: Post-incubation, the cells were analyzed for miR-23b expression via qPCR, and apoptosis was assessed using TUNEL staining and flow cytometry.
Data Collection and Analysis
Data on miR-23b expression levels were collected using qPCR, while apoptosis rates were quantified through flow cytometry and visualized via TUNEL staining. The results were statistically analyzed using one-way ANOVA to determine significance.
Results
Transfection of FP/miR-23b nanoparticles significantly restored miR-23b levels in LPS-stimulated macrophages, with a corresponding increase in apoptosis rates (37.44% in FP/miR-23b treated cells compared to control groups).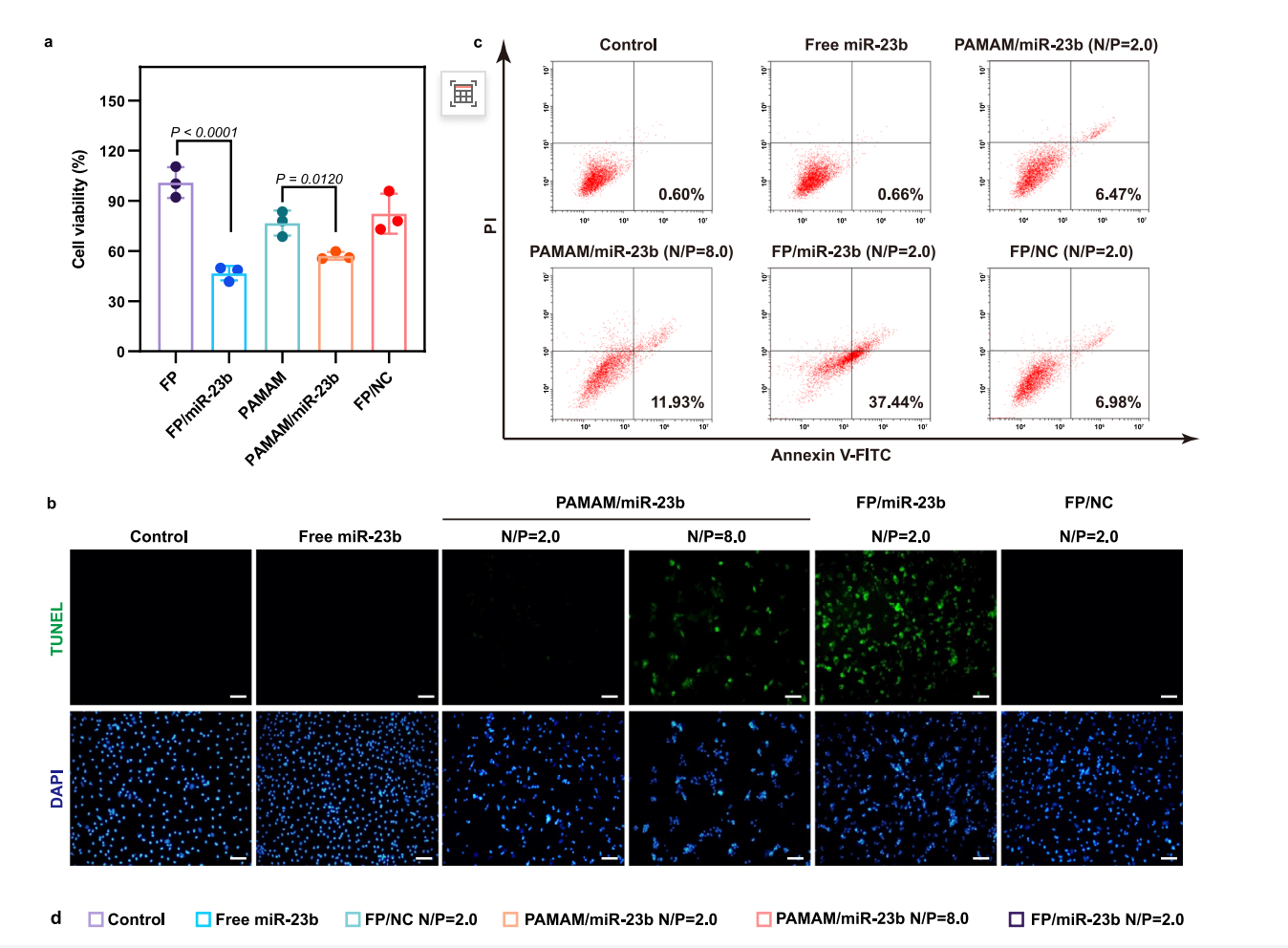
Figure 2. Inhibition of cell proliferation after miR-23b transfection in LPSstimulated BMDMs
Novel Aspects
The use of FP as a delivery vehicle demonstrated enhanced transfection efficiency in macrophages compared to traditional PAMAM or commercial reagents, providing a more effective strategy for treating chronic inflammation associated with RA.
In Vivo Therapeutic Efficacy Assessment
Key Steps
- Animal Model Preparation: Lewis rats were induced with adjuvant-induced arthritis (AIA) via subcutaneous injection of complete Freund’s adjuvant.
- Treatment Administration: Following the onset of arthritis symptoms, FP/miR-23b nanoparticles were administered intravenously at a dosage of 0.5 mg/kg body weight on specified days.
- Monitoring Clinical Parameters: Clinical assessments included measuring paw swelling, mobility tests (beam walking), and evaluation of joint health through X-ray and micro-CT imaging.
Data Collection and Analysis
Clinical data, including paw thickness and mobility scores, were recorded and analyzed over a 30-day period. Statistical analysis was performed using one-way ANOVA to compare the treatment effects among different groups.
Results
The administration of FP/miR-23b nanoparticles resulted in significantly reduced paw swelling and improved mobility compared to control groups. Radiological assessments showed a marked decrease in bone erosion and cartilage damage in treated rats.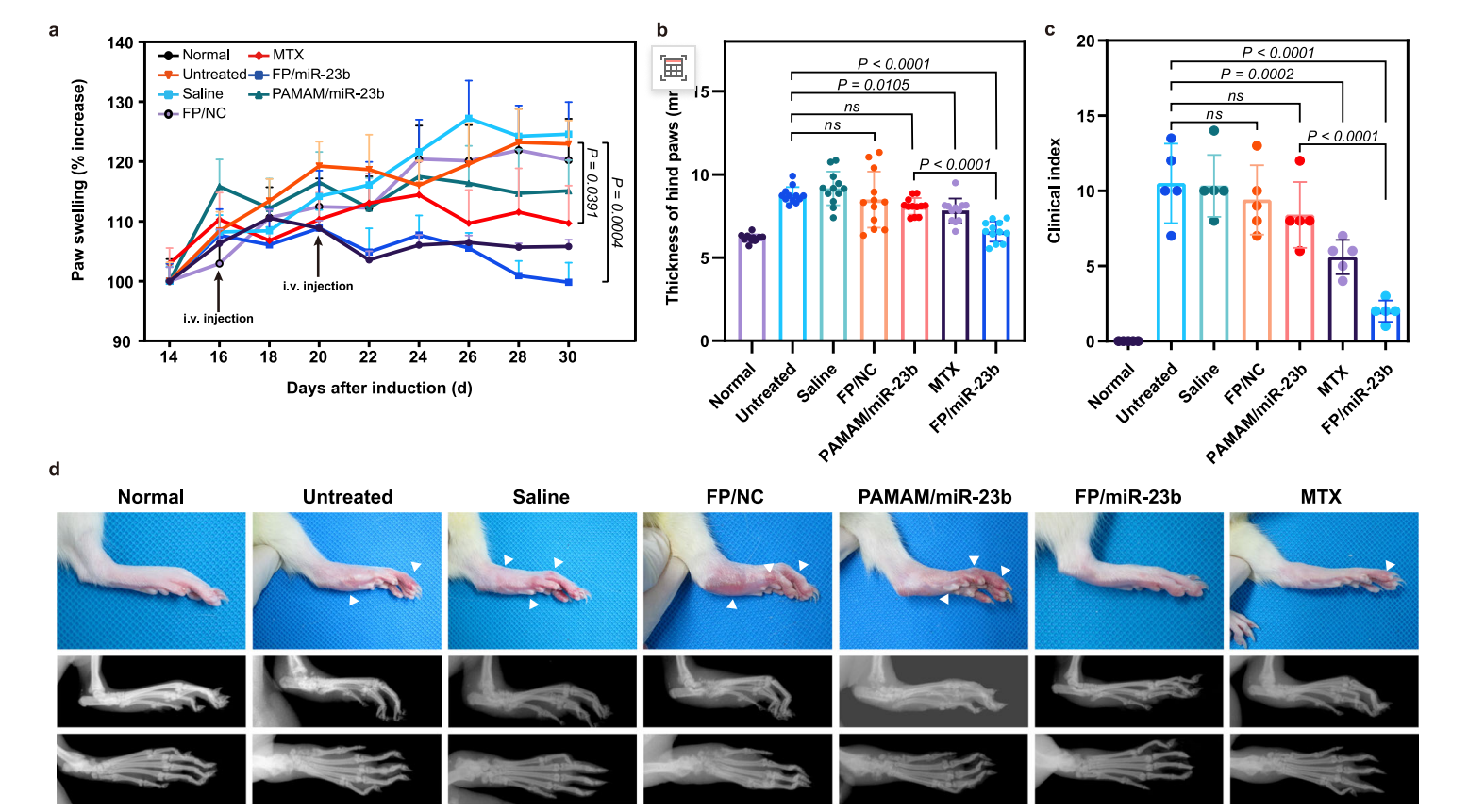
Figure 3. Therapeutic efficiency of FP/miR-23b nanoparticles in AIA rat model in terms of changes in inflamed paws
Novel Aspects
This study highlights the potential of systemic delivery of FP/miR-23b nanoparticles as a novel therapeutic approach for RA, demonstrating significant improvements in both clinical and radiological outcomes. The ability to target systemic inflammation effectively represents a significant advancement over traditional localized treatments.
Conclusion
The successful development of the fluorinated polyamidoamine dendrimer (FP) drug delivery system for the targeted delivery of microRNA-23b (miR-23b) was achieved through a combination of innovative synthesis techniques, rigorous in vitro and in vivo evaluations, and a robust understanding of the pathophysiology of rheumatoid arthritis (RA). By modifying the PAMAM dendrimer with hepta-fluorobutyric anhydride, the researchers enhanced the transfection efficiency and reduced the cytotoxicity typically associated with traditional gene delivery methods. This novel approach allowed for effective encapsulation and delivery of miR-23b directly to inflamed synovial tissues, thereby promoting localized therapeutic effects.
The highlights of the study include the demonstration that FP/miR-23b nanoparticles significantly reduced inflammation, improved mobility, and diminished bone damage in experimental RA models. Furthermore, the study illustrated the dual-action capability of miR-23b in inducing apoptosis in activated macrophages while simultaneously inhibiting inflammatory responses, presenting a promising strategy for more effective management of RA. Overall, this research not only provides insights into a novel therapeutic approach for autoimmune diseases but also sets the stage for future investigations into the broader applications of miRNA-based therapies.
Reference
Han, Haobo, et al. “Fluorinated polyamidoamine dendrimer-mediated miR-23b delivery for the treatment of experimental rheumatoid arthritis in rats.” Nature Communications 14.1 (2023): 944
