Editor: Tiffany
Researchers have unveiled an innovative multiplex inhalation platform, the Cloud α AX12, designed to accurately model aerosol delivery in a dynamic lung-on-chip environment, enhancing the study of inhalation toxicity and drug safety.
Key Highlights
- Research Question:
The study aimed to develop a reliable in vitro model for aerosol exposure that mimics the complex microenvironment of the distal lung to assess inhalation toxicity. - Research Difficulties:
Creating reproducible exposure models for the fragile and dynamic alveolar microenvironment posed significant challenges, as previous models had only focused on the upper airways. - Key Findings:
The Cloud α AX12 model effectively combined air-liquid interface and cyclic stretch conditions, revealing significant interactions between inhaled nanoparticles and the lung’s epithelial barriers, highlighting their inflammatory potential. - Innovative Aspects:
This study introduces a state-of-the-art lung-on-chip technology that integrates cloud-based aerosol exposure, allowing for more realistic simulation of in vivo inhalation scenarios. - Importance of the Study:
The findings are crucial for advancing inhalation drug development and toxicity assessments, providing a human-relevant alternative to animal testing amid growing regulatory pressures.
Background
Respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis affect millions globally, causing debilitating symptoms like shortness of breath and persistent cough. Current treatments, such as inhaled corticosteroids and bronchodilators, alleviate symptoms but often come with side effects and limited long-term efficacy. Preclinical testing for new inhalation therapies traditionally relies on animal models, which pose ethical concerns and frequently fail to predict human responses due to physiological differences. Conventional in vitro systems, while useful, lack the ability to replicate the lung’s dynamic environment, particularly the mechanical forces of breathing like cyclic stretch. These shortcomings highlight the urgent need for advanced in vitro platforms that can more accurately mimic the human lung’s complex physiology, improving the development of safer and more effective respiratory treatments.
Research Aim & Objectives
The primary challenge in inhalation research is creating reproducible in vitro models that reflect the human lung’s dynamic conditions, especially in the alveolar region. This requires simulating the air-liquid interface (ALI) and cyclic stretch (CS) of breathing while addressing the ethical and scientific limitations of animal testing. To meet this challenge, researchers Arunima Sengupta and Olivier T. Guenat from the University of Bern and AlveoliX AG developed the Cloud α AX12 platform. This innovative system integrates a breathing lung-on-chip with an aerosol exposure chamber to study aerosol delivery in a dynamic lung model. Published in Frontiers in Pharmacology on March 6, 2023, the study aimed to:
- Develop a physiologically relevant model combining ALI and CS.
- Validate uniform aerosol delivery and assess toxicity sensitivity.
- Test the platform’s ability to evaluate inhaled drug efficacy.
Research Methods & Results
Experimental Overview
- Development of the Cloud α AX12 Platform
- Validation of Aerosol Distribution
- Toxicity Assessment
- Therapeutic Efficacy Testing
- Data Analysis and Interpretation
The Cloud α AX12 platform was engineered by combining the VITROCELL cloud-based exposure chamber with the AlveoliX breathing AX Lung-on-chip system. The lung-on-chip component features an ultrathin, porous membrane that supports a multicellular model of the alveolar-capillary interface. This model incorporates human-derived alveolar epithelial cells, macrophages, and endothelial cells, cultured under ALI conditions with physiological CS to simulate breathing motions. The integration of these technologies enables the platform to recreate the complex cellular and mechanical environment of the distal lung. The researchers conducted several key experiments to evaluate the platform’s performance.
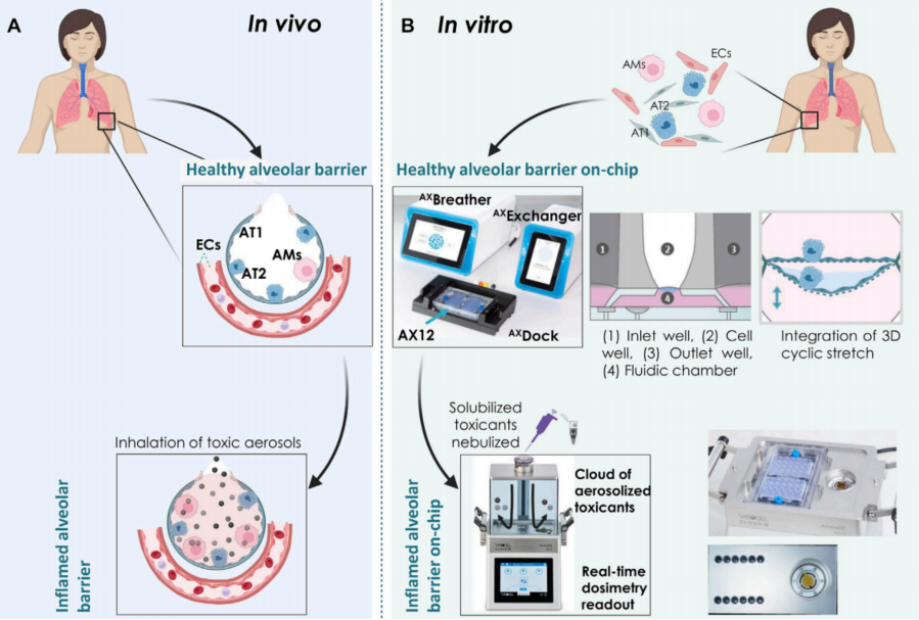
Figure 1. Overview of the Cloud α AX12 platform.
Key Experiments
- Validation of Aerosol Distribution
- Procedure: Researchers nebulized fluorescent tracers into the Cloud α AX12 system, then used imaging techniques to track their distribution across the lung-on-chip’s cell culture wells over multiple trials.
- Result: The tracers exhibited uniform deposition across all regions of the chip, with no significant variation, confirming the system’s ability to deliver aerosols consistently.
- New Finding: This uniform distribution is a critical advancement, ensuring that each well receives an identical aerosol dose, which enhances the reliability and reproducibility of subsequent toxicity and efficacy experiments compared to less consistent delivery methods.
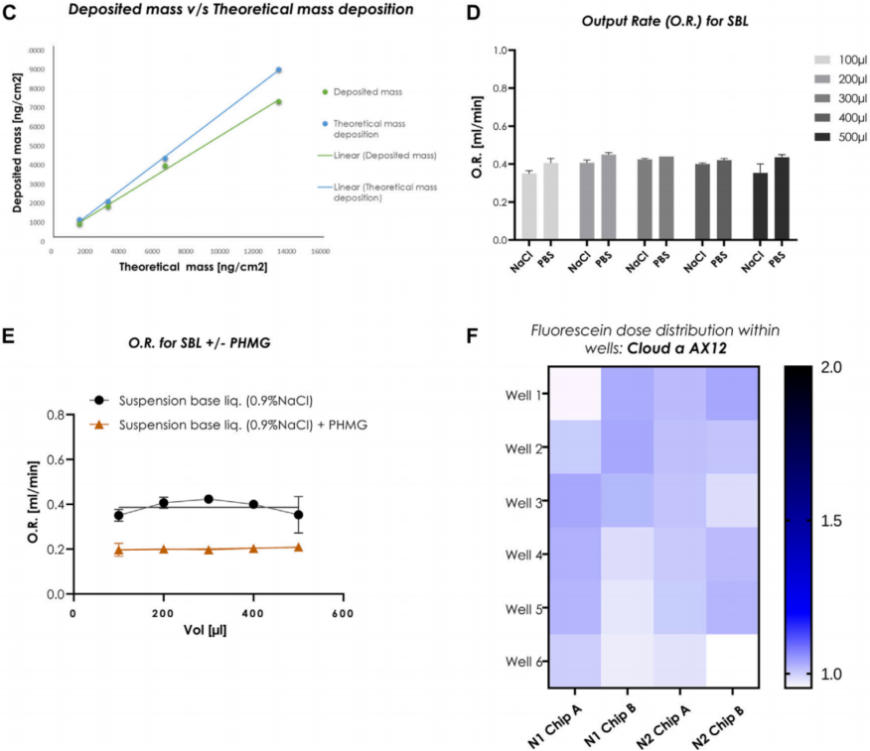
Figure 2. Homogenous dose distribution using the Cloud α AX12.
- Toxicity Assessment
- Procedure: The platform was exposed to titanium dioxide (TiO₂) and zinc oxide (ZnO) nanoparticles, as well as polyhexamethylene guanidine (PHMG). Cells were cultured under ALI, with some subjected to CS, and assessed for viability, barrier integrity, and inflammation markers over 24-48 hours.
- Result: Exposure caused significant barrier disruption, reduced cell viability, and elevated levels of inflammatory markers like IL-6 and TNF-α, with effects amplified under CS conditions compared to static ALI cultures.
- New Finding: The heightened sensitivity to toxicants under combined ALI and CS conditions reveals how mechanical forces exacerbate lung damage, offering a more realistic model of inhalation toxicity than traditional static systems.
- Therapeutic Efficacy Testing
- Procedure: Inflammation was induced with PHMG, followed by nebulization of the corticosteroid fluticasone propionate (FL) into the system. Researchers measured inflammatory cytokines and epithelial-mesenchymal transition (EMT) markers before and after treatment across multiple time points.
- Result: FL treatment significantly lowered cytokine levels (e.g., IL-6) and EMT markers, indicating reduced inflammation and tissue damage compared to untreated PHMG-exposed controls.
- New Finding: This experiment demonstrates the platform’s capability to assess inhaled drug efficacy accurately, providing a novel tool to evaluate therapeutic interventions in a human-relevant lung model, potentially speeding up drug development.
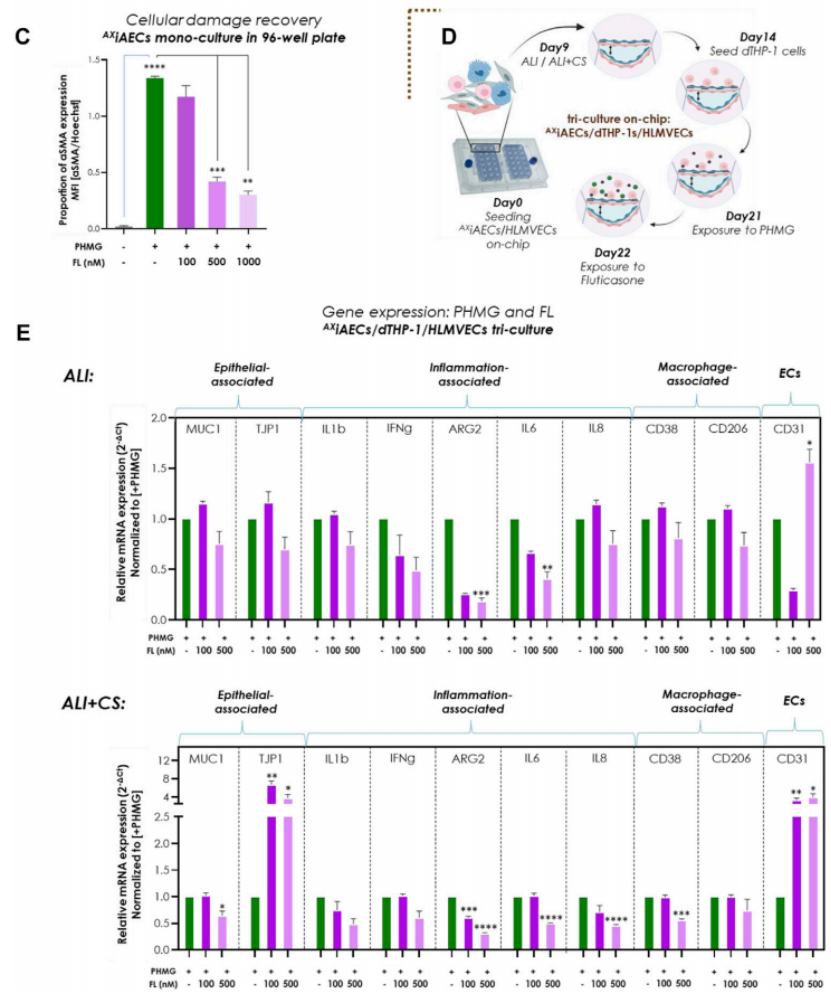
Figure 3. Inhaled Fluticasone reduced PHMG-induced inflammation and EMT.
Summary
The Cloud α AX12 platform advances inhalation research by integrating a breathing lung-on-chip with an aerosol exposure chamber, enabling the study of aerosol delivery in a dynamic, human-like lung model. Key findings include consistent aerosol distribution, increased toxicant sensitivity under ALI and CS conditions, and successful evaluation of fluticasone propionate’s therapeutic effects. By mimicking the lung’s mechanical and cellular environment, the platform offers a more predictive alternative to animal models, reducing ethical concerns while enhancing preclinical testing accuracy. Its ability to test both toxicity and drug efficacy positions it as a transformative tool for respiratory medicine, promising to accelerate the development of safer, more effective inhalation therapies.
Reference:
Sengupta, Arunima, et al. “A multiplex inhalation platform to model in situ like aerosol delivery in a breathing lung-on-chip.” Frontiers in Pharmacology 14 (2023): 1114739.
