Editor: Sarah
A recent study explores a new approach for the treatment of high-grade serous ovarian cancer (HGSOC), a particularly aggressive form of ovarian cancer. This study investigates the combination of two well-known cancer therapies: niraparib (a PARP inhibitor) and doxorubicin (a chemotherapy agent). By encapsulating both drugs into a nanoparticle formulation, the study aims to deliver the combination directly to cancer cells while minimizing systemic toxicity.
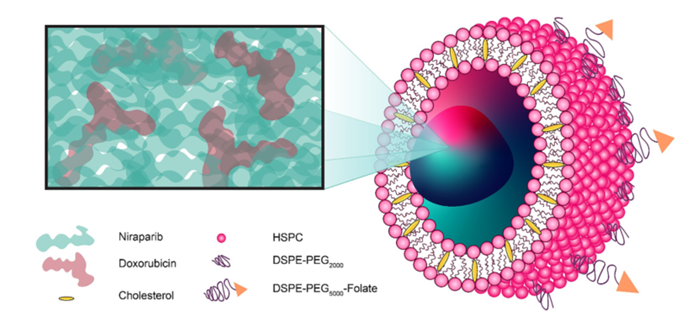
Figure 1: Schematic of the nanoparticle formulation.
Addressing Gaps in Ovarian Cancer Treatment
Ovarian cancer continues to be one of the leading causes of cancer-related deaths among women, with treatment outcomes improving only marginally in recent years. The current standard therapies, predominantly platinum-based, are often ineffective due to resistance and adverse side effects. This study aims to address these limitations by exploring a novel combination of niraparib and doxorubicin in a nanoparticle-based formulation, enhancing their therapeutic effects while mitigating toxicity.
PARP inhibitors like niraparib have shown potential in treating ovarian cancer, particularly in tumors with homologous recombination deficiencies (HRD), a common feature of HGSOC cells. However, the combination of PARP inhibitors with other chemotherapy agents often leads to significant toxicities. To overcome this challenge, the researchers encapsulated both drugs in a targeted nanoparticle formulation, thereby improving the drugs’ effectiveness and minimizing side effects.
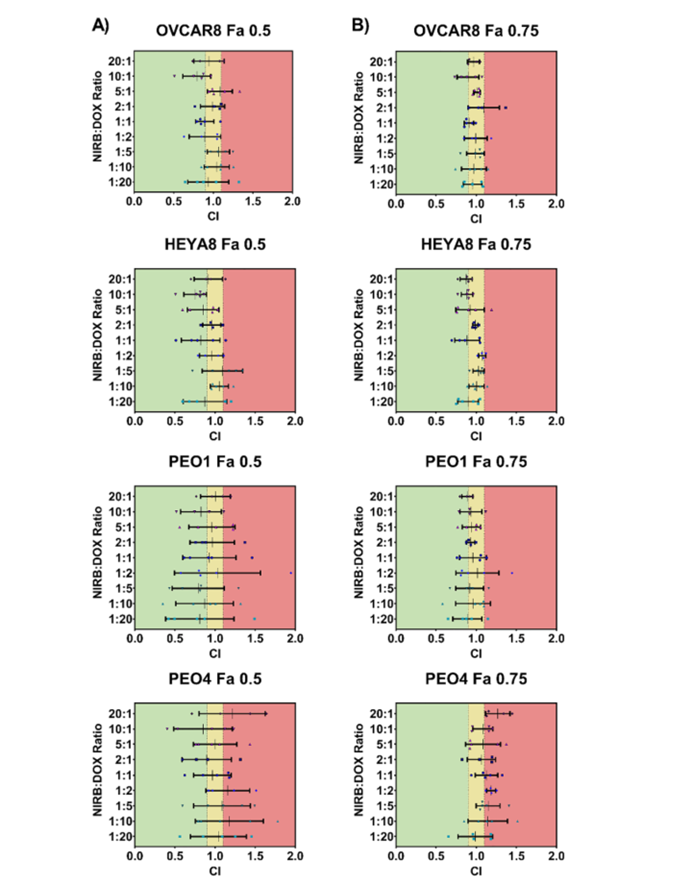
Figure 2: Synergy of Niraparib and Doxorubicin – a chart showing the combination index (CI) values for various molar ratios of niraparib and doxorubicin, demonstrating the synergy of a 10:1 ratio in different ovarian cancer cell lines.
Key Findings and Methodology
The study’s findings highlight that the 10:1 molar ratio of niraparib to doxorubicin exhibits consistent synergistic effects across multiple ovarian cancer cell lines, particularly in those with HR deficiencies. In contrast, the synergy diminishes in HR-proficient cells, which suggests that the effectiveness of the drug combination is influenced by the genetic profile of the cancer cells. The researchers successfully encapsulated this combination within a folate receptor-targeted nanoparticle, ensuring stable drug delivery, minimal toxicity, and controlled release.
Contributions and Key Findings:
- Drug Combination Synergy: The study found that a 10:1 molar ratio of niraparib to doxorubicin consistently produced synergistic effects across several ovarian cancer cell lines, particularly in those with HRD. However, in HR-proficient cell lines, the synergy decreased, suggesting that the combination therapy’s effectiveness may depend on the genetic profile of the tumor.
- In Vitro Screening: The researchers screened various molar ratios of niraparib and doxorubicin in multiple ovarian cancer cell lines using the Chou-Talalay method, which is commonly used to assess drug interactions. The 10:1 ratio was identified as the most effective for inducing synergy, particularly in HR-deficient cells.
- Nanoparticle Formulation: To overcome the toxicity issues associated with traditional chemotherapy, the drugs were encapsulated in folate receptor-targeted nanoparticles. These nanoparticles were designed to bind specifically to overexpressed folate receptors on cancer cells, ensuring precise drug delivery while reducing systemic side effects.
- Controlled Drug Release: The formulation enabled controlled release of both drugs, maintaining the desired 10:1 ratio for extended periods. This approach aims to provide sustained therapeutic effects with reduced toxicity.
- Cell Line Selection: Four ovarian cancer cell lines, including both HR-deficient and HR-proficient types, were used to evaluate the combination therapy. The study revealed that the 10:1 ratio consistently led to synergistic or additive effects in HR-deficient lines but resulted in antagonism in HR-proficient cell lines.
- Folate Receptor Targeting: The study also demonstrated that the folate receptor-targeted nanoparticles enhanced cellular uptake in ovarian cancer cells that overexpress folate receptors, such as OVCAR8 cells. This selective targeting ensures that the drugs are delivered more effectively to cancer cells, further minimizing the potential for side effects.
- Encapsulation Efficiency and Stability: The study used cryo-TEM imaging and other techniques to confirm the successful encapsulation of both drugs into the nanoparticles. The resulting drug-loaded liposomes exhibited high stability, ensuring that the drugs remain within the nanoparticle until they reach the target cells.
- Future Directions: While the findings are promising, further research is needed to refine the nanoparticle formulation and evaluate its effectiveness in animal models and clinical trials. Future studies should also explore the combination therapy’s response across a wider range of ovarian cancer cell lines, particularly those with diverse genetic mutations, to better understand the therapy’s potential applications.
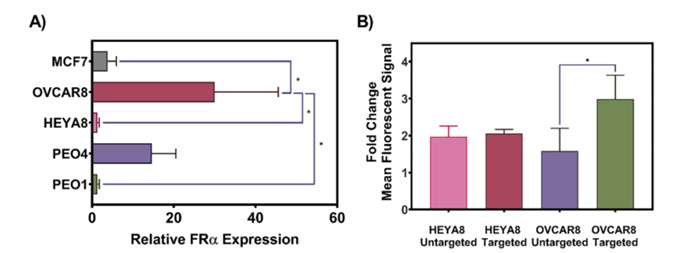
Figure 3: Cryo-TEM of Drug-Loaded Nanoparticles.
Implications for Ovarian Cancer Treatment
This nanoparticle formulation has the potential to significantly improve ovarian cancer treatment by offering a more efficient method of drug delivery. The targeted approach ensures that the combination therapy reaches the cancer cells with minimal systemic exposure, reducing harmful side effects. This method could be especially beneficial for patients with platinum-resistant or recurrent ovarian cancer, providing an alternative treatment option for these challenging cases.
Additionally, the controlled release of the drug payload could lead to prolonged therapeutic effects, which may improve clinical outcomes for patients. This dual-drug nanoparticle system, which has shown success in other cancer treatments, could pave the way for broader applications in other malignancies as well.
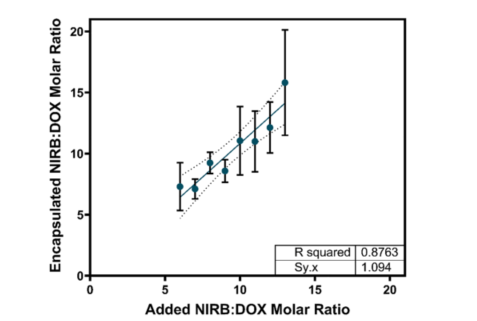
Figure 4: Targeted Delivery and Uptake.
Future Research and Clinical Applications
The study’s findings offer a foundation for further investigation into the use of combination therapies in nanoparticle formulations for ovarian cancer. Further research should focus on refining the formulation and testing its efficacy in animal models, as well as expanding clinical trials to assess its potential for broader patient populations. Personalized treatment strategies based on the genetic profiles of patients may help optimize the use of this combination therapy, ultimately improving patient outcomes and reducing unnecessary toxicities.
Reference
Wang, Lucy, et al. “Folate Receptor Targeted Nanoparticles Containing Niraparib and Doxorubicin as a Potential Candidate for the Treatment of High-Grade Serous Ovarian Cancer.” Scientific Reports, vol. 13, no. 3226, 2023, https://doi.org/10.1038/s41598-023-28424-3.
