Editor: Sarah
A recent study has introduced iron nitroprusside (FeNP) as a promising chemodynamic agent and inducer of ferroptosis in ovarian cancer therapy. This research, led by Kanwal Asif, Muhammad Adeel, Md. Mahbubur Rahman, and their colleagues, explores the potential of FeNP to address the challenges of current cancer treatments, especially in the case of ovarian cancer, a disease that continues to have limited treatment options and poor prognosis.
A New Approach to Cancer Treatment
The study primarily examines FeNP’s dual role: acting as a catalyst for the Fenton reaction and an inducer of ferroptosis, a unique form of cell death. The chemodynamic therapy (CDT) technique used in this study focuses on targeting oxidative stress to damage cancer cells. Previous CDT approaches have utilized metal ions to induce oxidative stress, but these often face challenges such as limited catalyst availability and the presence of antioxidant defenses in cancer cells, like glutathione peroxidase 4 (GPX4), which help cells survive oxidative stress. FeNP’s use overcomes these obstacles by providing a more efficient catalytic agent and inhibiting GPX4, positioning FeNP as a strong candidate for cancer therapy.
Experimental Findings and Methodology
FeNP, a dual metal iron-based compound, generates hydroxyl radicals (•OH) through the Fenton reaction, inducing cell death in ovarian cancer cells while exhibiting minimal toxicity toward normal cells. The research employed in vitro, ex vivo, and in vivo experiments to confirm the compound’s effectiveness.
Key findings from the study include:
- FeNP significantly reduced the growth of ovarian cancer cells, particularly in resistant strains like A2780cis, while showing minimal toxicity in normal cell lines.
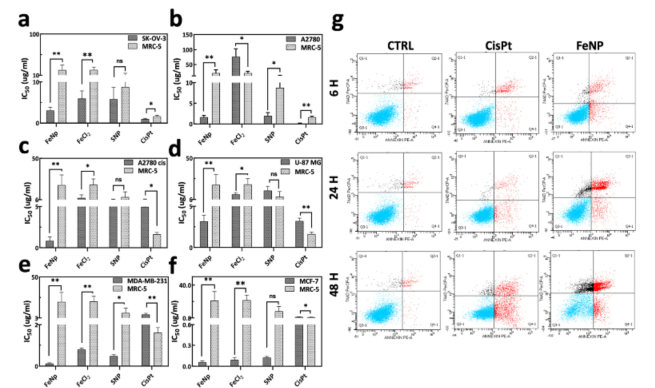
Figure 1: Cytotoxicity and Apoptosis Analysis of FeNP.
- The study confirmed the ability of FeNP to induce ferroptosis, as evidenced by the suppression of GPX4 activity over time, a key indicator of this type of cell death.
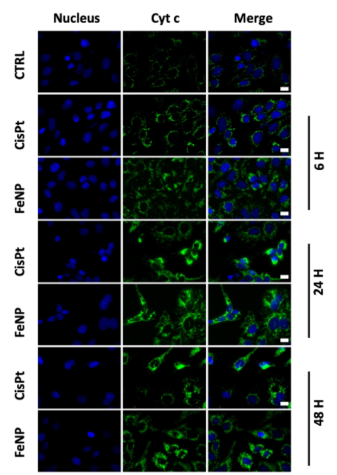
Figure 2: Cytochrome C Release, Lysosomal Internalization of FeNP.
- The results were supported by advanced techniques such as X-ray diffraction (XRD), Raman spectroscopy, and flow cytometry to assess FeNP’s stability, cellular uptake, and ability to induce cell death.
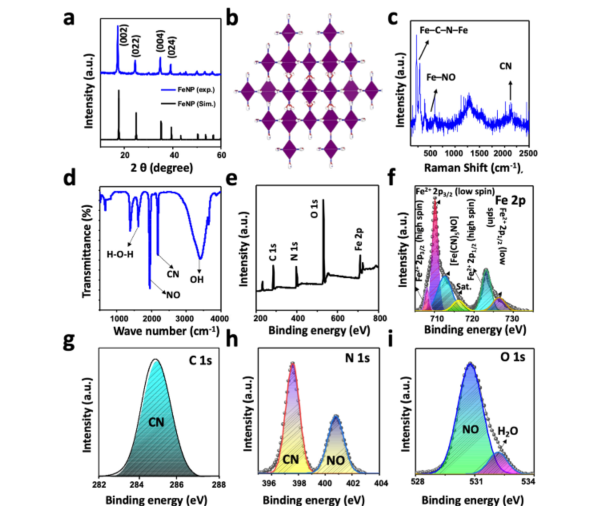
Figure 3: XRD Pattern, Raman, FTIR, and XPS Spectra of FeNP.
Implications for Ovarian Cancer Therapy
The study’s results suggest that FeNP could serve as a more effective and less toxic alternative to traditional chemotherapies. Its ability to induce ferroptosis in ovarian cancer cells without the need for external stimulation or delivery systems sets it apart from other therapies. Additionally, FeNP demonstrated biocompatibility in normal mouse liver organoids, supporting its potential for broader clinical application.
The therapeutic potential of FeNP is especially notable in ovarian cancer, which is resistant to many existing therapies like cisplatin. FeNP’s dual action—catalyzing oxidative stress and inhibiting GPX4—could be a key factor in improving cancer treatment.
Contributions and Key Findings
- Dual Mechanism of Action: FeNP acts both as a catalyst for the Fenton reaction, which produces highly reactive hydroxyl radicals (•OH) that damage cancer cells, and as an inducer of ferroptosis, a form of iron-dependent cell death.
- Efficient Catalysis of the Fenton Reaction: FeNP generates hydroxyl radicals through the Fenton reaction, leading to oxidative stress that significantly reduces ovarian cancer cell growth, particularly in resistant strains like A2780cis.
- Minimal Toxicity in Normal Cells: The compound showed minimal toxicity in normal cell lines, suggesting its potential for targeted cancer therapy with reduced side effects compared to conventional chemotherapies.
- GPX4 Inhibition and Ferroptosis Induction: FeNP inhibited the antioxidant enzyme GPX4 over time, which is critical in the induction of ferroptosis. This process was confirmed through a series of experiments, including Western blot analysis and flow cytometry.
- Cellular Uptake and Localization: FeNP was found to enter cells and accumulate in lysosomes, where it releases Fe2+ ions due to the acidic environment, leading to the generation of reactive oxygen species (ROS) and subsequent cancer cell death.
- Effectiveness in Ovarian Cancer Organoids: FeNP demonstrated effective therapeutic activity against ovarian cancer organoids, particularly patient-derived tumor organoids (PDTO) from high-grade serous ovarian cancer (HGSOC) patients, which are known to have poor survival rates.
- Biocompatibility in Normal Organoids: The compound showed biocompatibility with normal mouse liver organoids, with significantly lower toxicity compared to cisplatin, making it a safer option for potential therapeutic use.
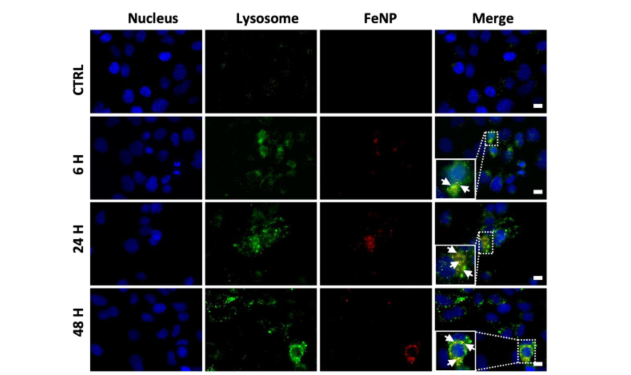
Figure 4: Histopathological Analysis and Anticancer Activity on Organoids.
- Preclinical In Vivo Efficacy: In animal studies, FeNP demonstrated a high therapeutic efficacy against ovarian cancer without significant adverse effects, confirming its potential as a promising candidate for clinical application.
Future Prospects
With further validation in clinical trials, FeNP has the potential to become an important agent in the development of more effective cancer treatments, particularly for cancers that exhibit resistance to traditional therapies. The study not only offers hope for improved ovarian cancer therapies but also suggests that FeNP may be applicable to other cancer types, providing new avenues for research in the field of cancer treatment.
Reference
Asif, Kanwal, et al. “Iron Nitroprusside as a Promising Agent for Ovarian Cancer Therapy.” Journal of Materials Chemistry B, vol. 11, 2023, pp. 3124-3135. The Royal Society of Chemistry, https://doi.org/10.1039/d2tb02691k.
