Editor: Tiffany
Key Highlights
- Research Question:
Can the combination of photodynamic therapy (PDT) and carbon monoxide (CO) release, triggered by near-infrared (NIR) light, enhance the therapeutic efficacy against glioblastoma? - Research Difficulties:
The study faced challenges related to the limited therapeutic efficacy of existing PDT approaches and the systemic toxicity associated with CO. - Key Findings:
The developed nanoplatform, UCNPs@Ce6/3HBQ@CM, demonstrated improved therapeutic efficacy by simultaneously generating CO and reactive oxygen species (ROS) in tumor sites, leading to enhanced cell apoptosis and reduced inflammation. - Innovative Aspects:
This is the first instance where both PDT and CO release are activated simultaneously via NIR light, allowing for deeper tissue penetration and targeted therapy. - Importance of the Study:
The research holds significant potential for improving treatment outcomes for glioblastoma patients, a condition known for its poor prognosis and high recurrence rates.
Scientists have developed an innovative treatment approach for glioblastoma (GBM), one of the deadliest forms of brain cancer, using advanced nanotechnology to combine two therapies in a single platform. This research, conducted by a team from Hubei University and published in the Journal of Nanobiotechnology in 2023, introduces a system that integrates photodynamic therapy (PDT) and carbon monoxide (CO) therapy, delivered via upconversion nanoparticles (UCNPs). The findings suggest this method could enhance the effectiveness of treatment for GBM, a cancer known for its poor prognosis and limited response to existing therapies.
Glioblastoma: A Formidable Challenge in Oncology
Glioblastoma is an aggressive, fast-growing tumor that originates in the brain and invades surrounding healthy tissue. This invasiveness makes it exceptionally challenging to treat. According to the research, patients diagnosed with GBM face a median survival time of only 12 to 15 months, even with the standard treatments of surgery, radiotherapy, and chemotherapy. The cancer’s high recurrence rate and resistance to these therapies contribute to a five-year survival rate of less than 10%. These statistics underscore the urgent need for new, more effective strategies to combat this devastating disease. Current treatments, while offering some benefit, often fail to prevent the cancer’s return or significantly extend patient survival, leaving both patients and doctors searching for better solutions.
A Novel Dual-Therapy Platform for GBM
The research team aimed to address these challenges by developing a novel therapeutic platform that combines PDT and CO therapy, activated by near-infrared (NIR) light. PDT is a treatment that uses light to activate a drug, called a photosensitizer, which then generates reactive oxygen species (ROS) to kill cancer cells. However, PDT has limitations, including poor penetration of light into deeper tissues and potential side effects such as inflammation. To overcome these drawbacks, the researchers incorporated CO therapy, which involves the controlled release of carbon monoxide—a molecule that, surprisingly, can enhance cancer treatment. CO interferes with the cancer cells’ energy production and reduces inflammation, potentially boosting the overall effectiveness of the therapy.
The innovation lies in the use of upconversion nanoparticles (UCNPs), tiny particles capable of absorbing NIR light and converting it into visible light. NIR light is advantageous because it can penetrate deeper into tissues compared to the light typically used in PDT. The visible light emitted by the UCNPs then activates two key components: a photosensitizer called Ce6 for PDT and a CO-releasing molecule known as 3HBQ. By integrating these two therapies into a single system, the researchers sought to create a more targeted and potent treatment for GBM, potentially overcoming some of the shortcomings of existing approaches.
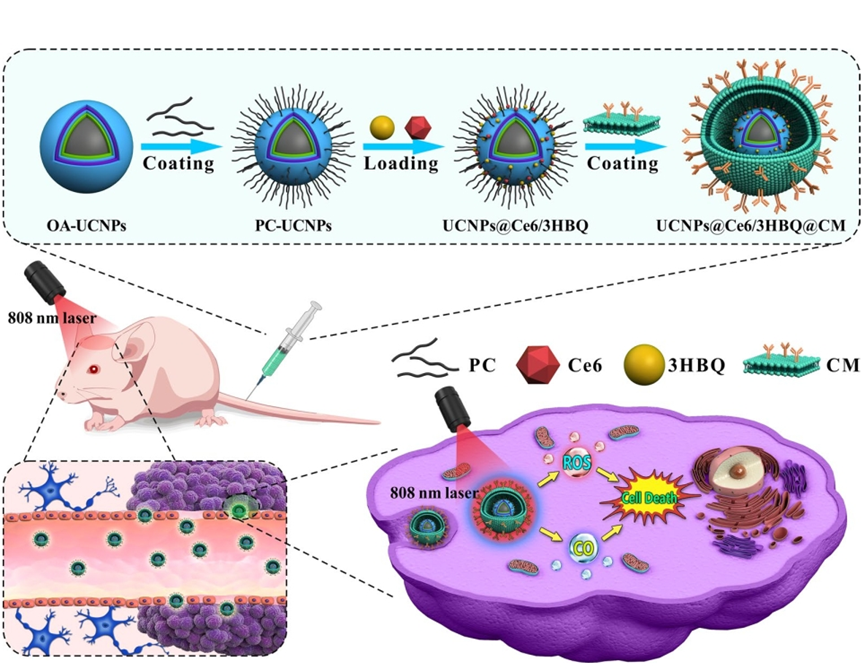
Figure 1. Schematic illustration of the synthesis process of lanthanide-doped nanoparticles loaded with photosesitizer Ce6 (chlorin e6) and photoCORM 3HBQ encapsulated by brain tumor cell membranes (UCNPs@Ce6/3HBQ@CM) and the anti-glioma mechanism (OA and PC represent oleic acid and phosphatidylcholine, respectively).
Experimental Validation of the Dual-Therapy Platform
To bring this concept to life, the researchers synthesized UCNPs with a specific structure designed to emit light at the precise wavelengths required to activate both Ce6 and 3HBQ. They loaded these nanoparticles with the photosensitizer and the CO-releasing molecule, creating a therapeutic nanocomposite. To improve targeting, the nanoparticles were coated with a layer of cell membrane derived from GBM cells, which helps the particles home in on the tumor site. This nanocomposite was then tested in two main experiments: one in cell cultures and another in mice with GBM tumors.
1. In Vitro Evaluation on Glioblastoma Cells
- Procedure: U87MG glioblastoma cells were treated with the nanocomposite and exposed to NIR light to activate both PDT and CO therapy.
- Result: The dual therapy induced apoptosis (programmed cell death) in 74.5% of the cells, compared to 52.3% with PDT alone.
- New Finding: The addition of CO therapy significantly enhances PDT’s cancer-killing effect, demonstrating the potential of this combined approach at the cellular level.
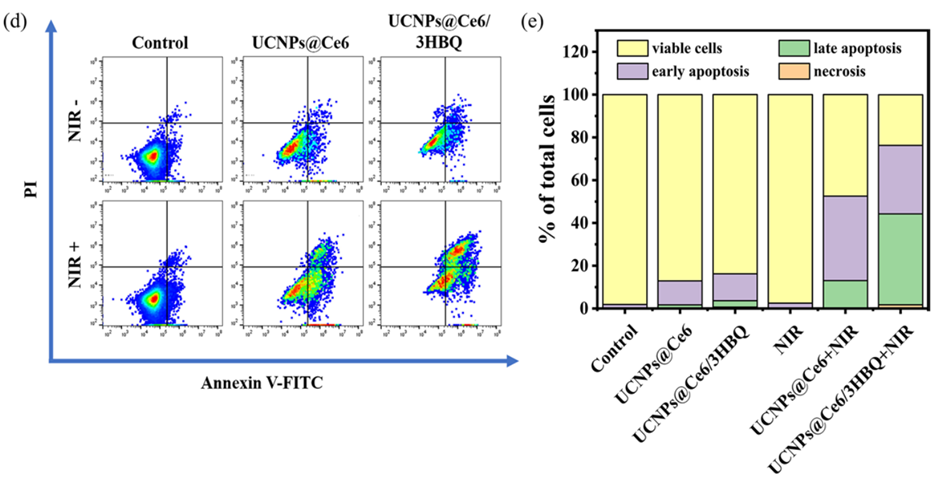
Figure 2. (d) Apoptosis analysis of U87MG cells after different treatments by flow cytometry. (e) Percentage of viable cells, early apoptosis, late apoptosis and necrosis obtained from (d)
2. In Vivo Evaluation in a Mouse Model of GBM
- Procedure: Mice with GBM tumors were injected with the nanocomposite and irradiated with NIR light at the tumor site.
- Result: Tumor growth was significantly reduced in mice receiving the dual therapy compared to those treated with PDT alone or controls, with improved survival rates observed.
- New Finding: The nanocomposite effectively targets and shrinks tumors in a living model, supporting its potential as a viable treatment strategy.
These results highlight the enhanced efficacy of combining PDT and CO therapy using UCNPs, both in vitro and in vivo.
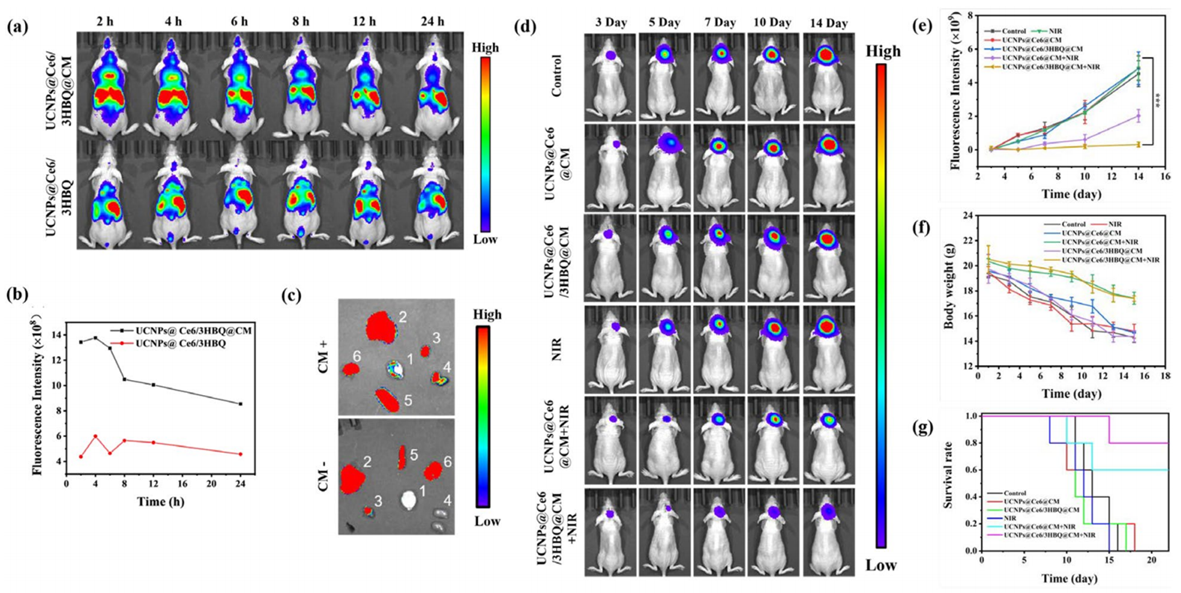
Figure 3. In vivo therapeutic efficiency of UCNPs@Ce6/3HBQ@CM.
Significance and Future Directions
The development of this nanocomposite marks a significant step forward in the fight against glioblastoma. By integrating PDT and CO therapy into a single, light-activated platform, the researchers have created a treatment that appears more effective than PDT alone, as evidenced by the increased cell death in lab tests and the reduced tumor growth in mice. The use of NIR light allows the therapy to reach deeper into the brain, targeting tumors that are difficult to access with conventional methods. Additionally, the inclusion of CO mitigates some of the inflammatory side effects associated with PDT, potentially making the treatment gentler on the body.
The study’s findings suggest that this approach could address some of the key limitations of current GBM treatments, such as their limited effectiveness and high recurrence rates. The ability to target the tumor directly with a dual-therapy system may offer a more precise and powerful alternative to surgery, radiotherapy, and chemotherapy. Moreover, the improved survival rates in the mouse model indicate that this platform has the potential to extend life expectancy for those affected by this aggressive cancer.
Reference:
Ge, Juan, et al. “Near-infrared light triggered in situ release of CO for enhanced therapy of glioblastoma.” Journal of Nanobiotechnology 21.1 (2023): 48.
